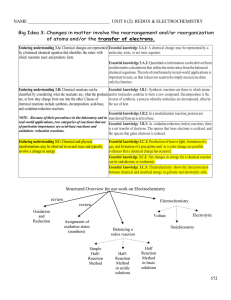
Kinetics and Equilibrium ___ 1. In a chemical reaction the use of a
... ___ 42. For a chemical system at equilibrium a rise in temperature will (1) favor the endothermic reaction; (2) favor the exothermic reaction; (3) decrease the rates of the reactions; (4) have no effect upon the equilibrium. ___ 43. The equilibrium constant for a given system changes when (1) the te ...
... ___ 42. For a chemical system at equilibrium a rise in temperature will (1) favor the endothermic reaction; (2) favor the exothermic reaction; (3) decrease the rates of the reactions; (4) have no effect upon the equilibrium. ___ 43. The equilibrium constant for a given system changes when (1) the te ...
analisis farmasi analisis farmasi anorganik -
... The ability of an aqueous solution to resist changes in pH upon the The ability of an aqueous solution to resist changes in pH upon the addition of acid or base is termed the buffering capability of the solution. The ability of a natural water body to resist a decrease in pH is very important due ...
... The ability of an aqueous solution to resist changes in pH upon the The ability of an aqueous solution to resist changes in pH upon the addition of acid or base is termed the buffering capability of the solution. The ability of a natural water body to resist a decrease in pH is very important due ...
IB Chemistry Online EQ_Ans
... Ethane molecules can only form weaker London (dispersion) forces with water molecules. Cholesterol has a polar alcohol group; however, it behaves as a nonpolar molecule due to the relatively large size of the ...
... Ethane molecules can only form weaker London (dispersion) forces with water molecules. Cholesterol has a polar alcohol group; however, it behaves as a nonpolar molecule due to the relatively large size of the ...
Electron attachment to molecular clusters by collisional charge transfer
... the difference in the ionization potential of the donor atom and the electron affinity of the acceptor molecule. In previous work, this electron transfer process has been used with fast alkali atom beams produced by charge exchange or sputtering to determine electron affinities for many molecules." ...
... the difference in the ionization potential of the donor atom and the electron affinity of the acceptor molecule. In previous work, this electron transfer process has been used with fast alkali atom beams produced by charge exchange or sputtering to determine electron affinities for many molecules." ...
The Free High School Science Texts: A Textbook for High School
... pictured the atom like a mini solar system where the electrons orbit the nucleus like planets orbiting around the sun. There were some problems with this model. For example it could not explain the very interesting observation that atoms only emit light at certain wavelengths or frequencies. Niels B ...
... pictured the atom like a mini solar system where the electrons orbit the nucleus like planets orbiting around the sun. There were some problems with this model. For example it could not explain the very interesting observation that atoms only emit light at certain wavelengths or frequencies. Niels B ...
Chemical Reactivities: Fundamental and Nuclear Reactions
... Matter: atoms are neither created nor destroyed in chemical reactions, only rearranged. So with this succinct introduction, let's begin balancing some simple chemical reactions. Probably the very best thing to remember about these is that if you make like atoms in compounds are ions and know their c ...
... Matter: atoms are neither created nor destroyed in chemical reactions, only rearranged. So with this succinct introduction, let's begin balancing some simple chemical reactions. Probably the very best thing to remember about these is that if you make like atoms in compounds are ions and know their c ...
Kinetics of Oxidation of Benzyl Alcohol with Dilute Nitric Acid
... oxidation of benzyl alcohol using molecular oxygen requires a heterogeneous catalyst, high temperature (210 °C), and longer reaction times (>5 h). Although the reported selectivity is quite good (75%-95%), the conversion levels are too low (maximum of 40%).14 Practically, in all the previously cited ...
... oxidation of benzyl alcohol using molecular oxygen requires a heterogeneous catalyst, high temperature (210 °C), and longer reaction times (>5 h). Although the reported selectivity is quite good (75%-95%), the conversion levels are too low (maximum of 40%).14 Practically, in all the previously cited ...
Chemistry II Exams and Keys Corrected 2016 Season
... oxidation reduction reactions, balancing redox reactions by oxidation state method, activity series, mole relationships, massmass problems¸ stoichiometry of redox solutions, solutions stoichiometry, electronic structure and periodic table/periodicity. FEBRUARY: chemical bonding, photon-electron spec ...
... oxidation reduction reactions, balancing redox reactions by oxidation state method, activity series, mole relationships, massmass problems¸ stoichiometry of redox solutions, solutions stoichiometry, electronic structure and periodic table/periodicity. FEBRUARY: chemical bonding, photon-electron spec ...
Enzyme Activity
... Therefore, in the presence of a competitive inhibitor, more substrate is needed to achieve Vmax: Competitive inhibitors do not alter Vmax. The effect of a competitive inhibitor is reversed by increasing ...
... Therefore, in the presence of a competitive inhibitor, more substrate is needed to achieve Vmax: Competitive inhibitors do not alter Vmax. The effect of a competitive inhibitor is reversed by increasing ...
Worksheet
... (E) 6 36. When hafnium metal is heated in an atmosphere of chlorine gas, the product of the reaction is found to contain 62.2 percent Hf by mass and 37.4 percent Cl by mass. What is the empirical formula for this compound? (A) HfCl (B) HfCl2 (C) HfCl3 (D) HfCl4 (E) Hf2Cl3 37. Which of the following ...
... (E) 6 36. When hafnium metal is heated in an atmosphere of chlorine gas, the product of the reaction is found to contain 62.2 percent Hf by mass and 37.4 percent Cl by mass. What is the empirical formula for this compound? (A) HfCl (B) HfCl2 (C) HfCl3 (D) HfCl4 (E) Hf2Cl3 37. Which of the following ...
FIRST-ROW TRANSITION METAL COMPLEXES OF OMEPRAZOLE
... Omeprazole (OME) is a proton pump inhibitor (PPI). PPIs have enabled to improve the treatment of various acid-peptic disorders. OME is a weak base and it can form several complexes with transition and non-transition metal ions. In the present paper, we are describing series of transition metal compl ...
... Omeprazole (OME) is a proton pump inhibitor (PPI). PPIs have enabled to improve the treatment of various acid-peptic disorders. OME is a weak base and it can form several complexes with transition and non-transition metal ions. In the present paper, we are describing series of transition metal compl ...
Intro to Titrimetry
... Ex: Volhard Methods and Fajans Method RedOx Titrations – involves species which undergo redox reactions. The reaction is monitored by RedOx indicators or thru Electrochemical methods. Ex: 2 MnO4- + 5 C2O42- + 16 H+ 2 Mn2+ + 10 CO2 + 8 H2O Complexometric Titrations – reactions involving chelating/c ...
... Ex: Volhard Methods and Fajans Method RedOx Titrations – involves species which undergo redox reactions. The reaction is monitored by RedOx indicators or thru Electrochemical methods. Ex: 2 MnO4- + 5 C2O42- + 16 H+ 2 Mn2+ + 10 CO2 + 8 H2O Complexometric Titrations – reactions involving chelating/c ...
8872 Chemistry H1 syllabus for 2016
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
Unit 8 Test Review
... Activity Series of Metals – an invaluable aid to predicting the products of replacement reactions. Each element on the list replaces from a compound any of the elements below it. The larger the interval between elements, the more vigorous the reaction. ...
... Activity Series of Metals – an invaluable aid to predicting the products of replacement reactions. Each element on the list replaces from a compound any of the elements below it. The larger the interval between elements, the more vigorous the reaction. ...
CfE HIGHER CHEMISTRY Chemistry in Society
... At the end of this reaction no reactants will remain but there will be 100% products. However, many important industrial reactants are reversible so we never achieve 100% products. Using balanced chemical equations along with data from industrial processes it is possible to determine how efficient a ...
... At the end of this reaction no reactants will remain but there will be 100% products. However, many important industrial reactants are reversible so we never achieve 100% products. Using balanced chemical equations along with data from industrial processes it is possible to determine how efficient a ...
program
... structural formulas of the components and the other way around: • polyesters; • polyamides. indicate what is understood by different types of isomers, recognise these and give examples of these: • structural isomers; • stereoisomers; • cis-trans isomers; • mirror image isomers. give the structural f ...
... structural formulas of the components and the other way around: • polyesters; • polyamides. indicate what is understood by different types of isomers, recognise these and give examples of these: • structural isomers; • stereoisomers; • cis-trans isomers; • mirror image isomers. give the structural f ...
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
127 - Chimica
... (CO),] (compound 4), identified spectroscopically (IR and 'H NMR), which was previously synthesized'" by photochemical hydrogenation of [Re2(CO)lo].The new method parallels that recently discovered8for the transformation of [Re4H6(CO)12]2into the unsaturated [Re4H5(CO),,]-. As in that case, the proc ...
... (CO),] (compound 4), identified spectroscopically (IR and 'H NMR), which was previously synthesized'" by photochemical hydrogenation of [Re2(CO)lo].The new method parallels that recently discovered8for the transformation of [Re4H6(CO)12]2into the unsaturated [Re4H5(CO),,]-. As in that case, the proc ...
AP Chemistry
... point of CCI4(I) (350 K) is higher than the normal boiling point of CF4(I) (145 K)? (A) The C-CI bonds in CCI4 are less polar than the C-F ...
... point of CCI4(I) (350 K) is higher than the normal boiling point of CF4(I) (145 K)? (A) The C-CI bonds in CCI4 are less polar than the C-F ...
College Chemistry I PHS 1025 Fall 2012 Practice Exam 3A
... B) C2H2 provides the most energy per gram and CH4 the least. C) CH4 provides the most energy per gram and C2H2 the least. D) C2H2 provides the most energy per gram and CH3OH the least. 28) Write a balanced net ionic equation for the reaction of AgNO3(aq) with KBr(aq). A) Ag+(aq) + NO3-(aq) + K+(aq) ...
... B) C2H2 provides the most energy per gram and CH4 the least. C) CH4 provides the most energy per gram and C2H2 the least. D) C2H2 provides the most energy per gram and CH3OH the least. 28) Write a balanced net ionic equation for the reaction of AgNO3(aq) with KBr(aq). A) Ag+(aq) + NO3-(aq) + K+(aq) ...
Document
... Pre-steady-state kinetics vs steady-state kinetics 1. The order of binding of substrates and release of product serves to define the reactants present at the active site during catalysis: it does not establish the kinetically preferred order of substrate addition and product release or allow conclu ...
... Pre-steady-state kinetics vs steady-state kinetics 1. The order of binding of substrates and release of product serves to define the reactants present at the active site during catalysis: it does not establish the kinetically preferred order of substrate addition and product release or allow conclu ...
In Class Overview of Chapter
... kinetics and thermodynamics are pretty distinct. Thermodynamics is about the equilibrium concentrations of products and reactants and tells us nothing about how fast equilibrium is reached. Kinetics tells us how fast a reaction will occur, but doesn’t tell us the extent or direction of a reaction. E ...
... kinetics and thermodynamics are pretty distinct. Thermodynamics is about the equilibrium concentrations of products and reactants and tells us nothing about how fast equilibrium is reached. Kinetics tells us how fast a reaction will occur, but doesn’t tell us the extent or direction of a reaction. E ...
Title Photo-Induced Electron Transfer Between a Reactant Molecule
... Catalytic alcohol oxidation to carbonyl compounds is one of the most important chemical transformations used in the industrial chemistry and in organic syntheses [51-53]. Oxidation of amines to imines is also an important chemical transformation because of the versatile applications of imines as syn ...
... Catalytic alcohol oxidation to carbonyl compounds is one of the most important chemical transformations used in the industrial chemistry and in organic syntheses [51-53]. Oxidation of amines to imines is also an important chemical transformation because of the versatile applications of imines as syn ...
Photoredox catalysis
_Schematic.png?width=300)
Photoredox catalysis is a branch of catalysis that harnesses the energy of visible light to accelerate a chemical reaction via a single-electron transfer. This area is named as a combination of ""photo-"" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that otherwise would not react. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes and semiconductors. While each class of materials has advantages, soluble transition-metal complexes are used most often.Study of this branch of catalysis led to the development of new methods to accomplish known and new chemical transformations. One attraction to the area is that photoredox catalysts are often less toxic than other reagents often used to generate free radicals, such as organotin reagents. Furthermore, while photoredox catalysts generate potent redox agents while exposed to light, they are innocuous under ordinary conditions Thus transition-metal complex photoredox catalysts are in some ways more attractive than stoichiometric redox agents such as quinones. The properties of photoredox catalysts can be modified by changing ligands and the metal, reflecting the somewhat modular nature of the catalyst.While photoredox catalysis has most often been applied to generate known reactive intermediates in a novel way, the study of this mode of catalysis led to the discovery of new organic reactions, such as the first direct functionalization of the β-arylation of saturated aldehydes. Although the D3-symmetric transition-metal complexes used in many photoredox-catalyzed reactions are chiral, the use of enantioenriched photoredox catalysts led to low levels of enantioselectivity in a photoredox-catalyzed aryl-aryl coupling reaction, suggesting that the chiral nature of these catalysts is not yet a highly effective means of transmitting stereochemical information in photoredox reactions. However, while synthetically useful levels of enantioselectivity have not been achieved using chiral photoredox catalysts alone, optically-active products have been obtained through the synergistic combination of photoredox catalysis with chiral organocatalysts such as secondary amines and Brønsted acids.