5 - BrainMass
... smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? b. Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain. ...
... smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? b. Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain. ...
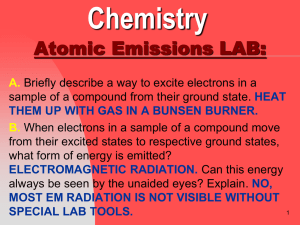
Atomic Emissions LAB Questions
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
ORDANOCHROMIUM CHEMISTRY SUPPORTED BY -DIIMINE LIGANDS
... α-Diimine ligands can accept up to two electrons; thus they can be used to stabilize organometallic compounds in unusually low formal oxidation states of the central metal. This redox ambiguity may be useful for facilitating catalytic reactions involving different oxidation states. We are exploring ...
... α-Diimine ligands can accept up to two electrons; thus they can be used to stabilize organometallic compounds in unusually low formal oxidation states of the central metal. This redox ambiguity may be useful for facilitating catalytic reactions involving different oxidation states. We are exploring ...
Photosynthesis Stores Energy in Organic Compounds
... I absorbs light energy while steps 1 & 2 are taking place Electron is excited, moves to a high-energy electron acceptor Excited electron is replaced by an electron from the end of the electron transport system ...
... I absorbs light energy while steps 1 & 2 are taking place Electron is excited, moves to a high-energy electron acceptor Excited electron is replaced by an electron from the end of the electron transport system ...
Chem 400 Chem 150 REVIEW SHEET Amanda R
... o Electron Affinity/Ionization Energy and electronegativity increases going up and to the right Types of Bonds – must know which bond types can form and how o Covalent o Ionic o Molecular o Bond order # of bonding e- - # of antibonding e-/2 Stoichiometry – must be able to balance reactions for any u ...
... o Electron Affinity/Ionization Energy and electronegativity increases going up and to the right Types of Bonds – must know which bond types can form and how o Covalent o Ionic o Molecular o Bond order # of bonding e- - # of antibonding e-/2 Stoichiometry – must be able to balance reactions for any u ...
Photosynthesis Stores Energy in Organic Compounds
... which moves to a higher energy level electron passes to the electron-accepting molecule. • After this, the electron moves through 4 steps ...
... which moves to a higher energy level electron passes to the electron-accepting molecule. • After this, the electron moves through 4 steps ...
Sugárkémiai áttekintés Schiller Róbert
... One must know the activity of the source, then Delementary must be integrated over source and irradiated space. ...
... One must know the activity of the source, then Delementary must be integrated over source and irradiated space. ...
Review 1st Qtr KEY
... a. an s orbital. c. a combination of px and py orbitals. b. a px orbital. d. a combination of an s and a px orbital. ...
... a. an s orbital. c. a combination of px and py orbitals. b. a px orbital. d. a combination of an s and a px orbital. ...
Chapter 18 Review 18.1 Oxidation-Reduction Reactions Oxidation
... where the agents are separated, the electrons flow through a wire, and there is a salt bridge connecting the two solutions Anode- the electrode where oxidation occurs Cathode- the electrode where reduction occurs Electrolysis- electrical energy is used to produce a chemical change - batteries uses e ...
... where the agents are separated, the electrons flow through a wire, and there is a salt bridge connecting the two solutions Anode- the electrode where oxidation occurs Cathode- the electrode where reduction occurs Electrolysis- electrical energy is used to produce a chemical change - batteries uses e ...
How to Assign Oxidation Numbers
... What is reduction? When a molecule/ion gains electrons (becomes more negative) Whatever is reduced is the oxidizing agent LEO GER ...
... What is reduction? When a molecule/ion gains electrons (becomes more negative) Whatever is reduced is the oxidizing agent LEO GER ...
Lecture 20 The Redox Sequence
... Because the energy of the sun is trapped in the C-C bonds, bacteria are indirectly using sunlight when they combust natural organic matter to CO2. Bacteria use the electron acceptors in the order of decreasing energy availability. ...
... Because the energy of the sun is trapped in the C-C bonds, bacteria are indirectly using sunlight when they combust natural organic matter to CO2. Bacteria use the electron acceptors in the order of decreasing energy availability. ...
Bohr Model of Hydrogen
... • (n = 1) is the ground state. This is the lowest energy state. E = -13.6 eV. • Whenever an electron is in any level above n = 1, the atom is “excited.” • Whenever an electron gains 13.6 eV, the electron is excited so much it is removed from the atom and an ion is formed. ...
... • (n = 1) is the ground state. This is the lowest energy state. E = -13.6 eV. • Whenever an electron is in any level above n = 1, the atom is “excited.” • Whenever an electron gains 13.6 eV, the electron is excited so much it is removed from the atom and an ion is formed. ...
Organometallic Compounds and Catalysis: Synthesis
... Organometallic Compounds and Catalysis: Synthesis and Use of Wilkinson’s Catalyst Organometallic chemistry is the chemistry of compounds which contain a metal carbon bond. Research interest in this area is largely fueled by potential applications of organometallic compounds as catalysts in industria ...
... Organometallic Compounds and Catalysis: Synthesis and Use of Wilkinson’s Catalyst Organometallic chemistry is the chemistry of compounds which contain a metal carbon bond. Research interest in this area is largely fueled by potential applications of organometallic compounds as catalysts in industria ...
Midterm Exam 2
... molecule and then to solve the Schrödinger equation for the electrons at that nuclear separation. This can be illustrated with a molecular potential energy curve like the one shown below. In this diagram what does the bottom of the well represent? The point where the dotted lines intersect. ...
... molecule and then to solve the Schrödinger equation for the electrons at that nuclear separation. This can be illustrated with a molecular potential energy curve like the one shown below. In this diagram what does the bottom of the well represent? The point where the dotted lines intersect. ...
Notes
... Another way of looking at it is that one species causes the electron loss or gain. A species that is being oxidized causes the other species to gain electrons and be reduced. A substance that is reduced acts as an _________________________ agent, while a substance that is oxidized acts as a ________ ...
... Another way of looking at it is that one species causes the electron loss or gain. A species that is being oxidized causes the other species to gain electrons and be reduced. A substance that is reduced acts as an _________________________ agent, while a substance that is oxidized acts as a ________ ...
Culver City H.S. • AP Chemistry Name Period ___ Date ___/___/___
... It takes more energy to ionize (completely remove) the electron from n=3 than from the ground state. The electron is farther from the nucleus on average in the n=3 state than in the n=1 state. The wavelength of light emitted if the electron drops from n=3 to n=2 will be shorter than the wavelength o ...
... It takes more energy to ionize (completely remove) the electron from n=3 than from the ground state. The electron is farther from the nucleus on average in the n=3 state than in the n=1 state. The wavelength of light emitted if the electron drops from n=3 to n=2 will be shorter than the wavelength o ...
Title - Iowa State University
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
Practical, Asymmetric Redox-Neutral Chemical Synthesis via Borrowing Hydrogen
... Synthetic chemistry is considered to be the “central science” as it provides the toolbox for various applied areas such as pharmaceuticals, chemical biology and material science. Due to resource constraints, the current trend in synthetic chemistry is not simply about preparing molecules of specific ...
... Synthetic chemistry is considered to be the “central science” as it provides the toolbox for various applied areas such as pharmaceuticals, chemical biology and material science. Due to resource constraints, the current trend in synthetic chemistry is not simply about preparing molecules of specific ...
Chemistry 212 Name:
... Each halogen is obtained by oxidation of the halide ion to the halogen in a molten salt, except fluorine. None of the halogens is particularly abundant in nature, however all are easily accessible in concentrated forms rendering this point moot. All halogens have high electron affinities and ionizat ...
... Each halogen is obtained by oxidation of the halide ion to the halogen in a molten salt, except fluorine. None of the halogens is particularly abundant in nature, however all are easily accessible in concentrated forms rendering this point moot. All halogens have high electron affinities and ionizat ...
Oxidation-Reduction (Redox) Reactions
... Balancing Redox Reactions Step 1: Break the unbalanced equation into half-reactions. Step 2: Balance each half-reaction by following a-d. a. Balance all elements except O and H by adjusting coefficients. b. Balance O by adding H2O to the appropriate side. c. Balance H by adding the appropriate numb ...
... Balancing Redox Reactions Step 1: Break the unbalanced equation into half-reactions. Step 2: Balance each half-reaction by following a-d. a. Balance all elements except O and H by adjusting coefficients. b. Balance O by adding H2O to the appropriate side. c. Balance H by adding the appropriate numb ...
Nugget
... David E. Lewis, Department of Chemistry, University of Wisconsin-Eau Claire, Eau Claire, WI 54702 The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the invers ...
... David E. Lewis, Department of Chemistry, University of Wisconsin-Eau Claire, Eau Claire, WI 54702 The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the invers ...
(s) If 5.00 moles of zinc is placed into 1.50 L... 34. solution,what is the mass of the hydrogen gas produced?
... Zn (s) + 2 HCI (aq) ~ Znc~ (s) +Hz(g) If 5.00 moles of zinc is placed into 1.50 L of a 3.00MHCI 34. solution,what is the mass of the hydrogen gas produced? (A) 0.750 g (D) 5.00 g (B) 2.25 g (E) 10.0 g (C) 4.50 g 32. How many grams of Zinc (atomic mass 65.0 g) are required to react completelywith 1.0 ...
... Zn (s) + 2 HCI (aq) ~ Znc~ (s) +Hz(g) If 5.00 moles of zinc is placed into 1.50 L of a 3.00MHCI 34. solution,what is the mass of the hydrogen gas produced? (A) 0.750 g (D) 5.00 g (B) 2.25 g (E) 10.0 g (C) 4.50 g 32. How many grams of Zinc (atomic mass 65.0 g) are required to react completelywith 1.0 ...
Project Details PPT
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
Photoredox catalysis
_Schematic.png?width=300)
Photoredox catalysis is a branch of catalysis that harnesses the energy of visible light to accelerate a chemical reaction via a single-electron transfer. This area is named as a combination of ""photo-"" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that otherwise would not react. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes and semiconductors. While each class of materials has advantages, soluble transition-metal complexes are used most often.Study of this branch of catalysis led to the development of new methods to accomplish known and new chemical transformations. One attraction to the area is that photoredox catalysts are often less toxic than other reagents often used to generate free radicals, such as organotin reagents. Furthermore, while photoredox catalysts generate potent redox agents while exposed to light, they are innocuous under ordinary conditions Thus transition-metal complex photoredox catalysts are in some ways more attractive than stoichiometric redox agents such as quinones. The properties of photoredox catalysts can be modified by changing ligands and the metal, reflecting the somewhat modular nature of the catalyst.While photoredox catalysis has most often been applied to generate known reactive intermediates in a novel way, the study of this mode of catalysis led to the discovery of new organic reactions, such as the first direct functionalization of the β-arylation of saturated aldehydes. Although the D3-symmetric transition-metal complexes used in many photoredox-catalyzed reactions are chiral, the use of enantioenriched photoredox catalysts led to low levels of enantioselectivity in a photoredox-catalyzed aryl-aryl coupling reaction, suggesting that the chiral nature of these catalysts is not yet a highly effective means of transmitting stereochemical information in photoredox reactions. However, while synthetically useful levels of enantioselectivity have not been achieved using chiral photoredox catalysts alone, optically-active products have been obtained through the synergistic combination of photoredox catalysis with chiral organocatalysts such as secondary amines and Brønsted acids.