The Elements of Group 15 (5A, V, VA) The Nitrogen Group
... Valence electron configuration: ns2np3 ...
... Valence electron configuration: ns2np3 ...
Oxidation Numbers
... reduction: a decrease in the oxidation number Cl2(g) + 2e− → 2Cl−(aq) Sn4+(aq) + 2e− → Sn2+(aq) (oxidation # becoming less positive or more negative) ...
... reduction: a decrease in the oxidation number Cl2(g) + 2e− → 2Cl−(aq) Sn4+(aq) + 2e− → Sn2+(aq) (oxidation # becoming less positive or more negative) ...
Coordination Chemistry of Life Processes: Bioinorganic Chemistry
... (iii) catalytic roles in oxidation-reduction (including oxygenation) reactions and (iv) catalytic roles in acid-base and other reactions. It has also been known for a long time that excesses of these elements can be very dangerous. In fact, a narrow concentration window exists for most of the so-cal ...
... (iii) catalytic roles in oxidation-reduction (including oxygenation) reactions and (iv) catalytic roles in acid-base and other reactions. It has also been known for a long time that excesses of these elements can be very dangerous. In fact, a narrow concentration window exists for most of the so-cal ...
SAMPLE QUESTION PAPER-II Chemistry (Theory) Class-XII
... They prepare benzene diazonium chloride and stored it at room temperature. Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again and to use it immediately to prepare azo dye and they proceed accordingly an ...
... They prepare benzene diazonium chloride and stored it at room temperature. Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again and to use it immediately to prepare azo dye and they proceed accordingly an ...
Chapter 4 (Hill/Petrucci/McCreary/Perry Chemical Reactions in
... This chapter deals with reactions that occur in aqueous solution …these solutions all use water as the solvent. We will look at some properties of these solutions and also look briefly at three different general types of reactions that occur in aqueous solutions. “water is such a good solvent for so ...
... This chapter deals with reactions that occur in aqueous solution …these solutions all use water as the solvent. We will look at some properties of these solutions and also look briefly at three different general types of reactions that occur in aqueous solutions. “water is such a good solvent for so ...
CH 5-7 Chapter 5-7 review wkey
... 35. Consider the thermal energy transfer during a chemical process. When heat is transferred to the system, the process is said to be _______ and the sign of H is ________. a) exothermic, positive b) endothermic, negative c) exothermic, negative d) endothermic, positive ...
... 35. Consider the thermal energy transfer during a chemical process. When heat is transferred to the system, the process is said to be _______ and the sign of H is ________. a) exothermic, positive b) endothermic, negative c) exothermic, negative d) endothermic, positive ...
+ H 2 SO 4(aq) - Rothschild Science
... element you have NH3 (one nitrogen, three hydrogen)- DON’T mess with these!! Coefficients – small whole number that appears ...
... element you have NH3 (one nitrogen, three hydrogen)- DON’T mess with these!! Coefficients – small whole number that appears ...
word doc (perfect formatting)
... 1) Represents an atom that is in an excited state 2) Represents an atom that is a noble gas 3) Represents an atom that is a transition metal 4) Represents an atom of an alkali earth metal Questions 5-8 refer to the following descriptions of bonding in different types of solids. a) Lattice of positiv ...
... 1) Represents an atom that is in an excited state 2) Represents an atom that is a noble gas 3) Represents an atom that is a transition metal 4) Represents an atom of an alkali earth metal Questions 5-8 refer to the following descriptions of bonding in different types of solids. a) Lattice of positiv ...
File - Mr. J`s Chemistry 4U
... Zinc atoms have a greater tendency to lose electrons than do copper atoms. Aluminum can replace zinc. Cobalt can replace sodium. Flourine is the most active halogen. Any metal above magnesium replaces hydrogen from water. Any metal above hydrogen reacts with acids, replacing hydrogen. Elements near ...
... Zinc atoms have a greater tendency to lose electrons than do copper atoms. Aluminum can replace zinc. Cobalt can replace sodium. Flourine is the most active halogen. Any metal above magnesium replaces hydrogen from water. Any metal above hydrogen reacts with acids, replacing hydrogen. Elements near ...
CHEM1405 2012-J-2 June 2012 • What is the ground state electron
... Reorient the molecule so that the lowest priority group (H) is at the back. Viewing down the C–H bond, the orientation of NH3+ → CO2– → CH2Ph is anticlockwise. Therefore (L)-phenylalanine has (S) configuration. ...
... Reorient the molecule so that the lowest priority group (H) is at the back. Viewing down the C–H bond, the orientation of NH3+ → CO2– → CH2Ph is anticlockwise. Therefore (L)-phenylalanine has (S) configuration. ...
Oxidation-Reduction Reactions Oxidation-Reduction
... amount of substance A by adding a carefully measured volume of a solution with known concentration of B until the reaction of A and B is just complete. (See Figure 4.20) Volumetric analysis is a method of analysis based on titration. ...
... amount of substance A by adding a carefully measured volume of a solution with known concentration of B until the reaction of A and B is just complete. (See Figure 4.20) Volumetric analysis is a method of analysis based on titration. ...
Cl Cl and
... 27. Why don’t elements of group 4 form ions of charge 4+? Why don’t they form ions of charge 4–? Too much energy is needed to remove 4 electrons from an atom. Too much energy is needed to insert 4 electrons into an atom in order to overcome the repulsive forces between like charges. 28. Why do eleme ...
... 27. Why don’t elements of group 4 form ions of charge 4+? Why don’t they form ions of charge 4–? Too much energy is needed to remove 4 electrons from an atom. Too much energy is needed to insert 4 electrons into an atom in order to overcome the repulsive forces between like charges. 28. Why do eleme ...
honors chem 6 day review packet
... Calculate the % concentration by mass of a solution in which 20 g Mg(OH)2 is dissolved in 80 g H2O. ...
... Calculate the % concentration by mass of a solution in which 20 g Mg(OH)2 is dissolved in 80 g H2O. ...
09 Stoichiometry WS Stoichiometry WS
... 10. A car battery produces electrical energy with the following chemical reaction: Pb(s) + PbO2(s) + 2H2SO4(aq) 2PbSO4(s) + 2H2O(l) If the battery loses 340. g of lead in this reaction, how many moles of lead(II) sulfate are produced? 11. In a space shuttle, the CO2 that the crew exhales is removed ...
... 10. A car battery produces electrical energy with the following chemical reaction: Pb(s) + PbO2(s) + 2H2SO4(aq) 2PbSO4(s) + 2H2O(l) If the battery loses 340. g of lead in this reaction, how many moles of lead(II) sulfate are produced? 11. In a space shuttle, the CO2 that the crew exhales is removed ...
Chemical Reactions
... There are many kinds of chemical reactions and several ways to classify them. One useful method of classifies reactions into four major types. These are: 1.) synthesis; 2.) decomposition; 3.) single replacement; and 4.) double replacement reactions. Not all reactions can be put into one of these cat ...
... There are many kinds of chemical reactions and several ways to classify them. One useful method of classifies reactions into four major types. These are: 1.) synthesis; 2.) decomposition; 3.) single replacement; and 4.) double replacement reactions. Not all reactions can be put into one of these cat ...
First of all, do you know any methods to check
... Error in AES: analysis: < 15%, Error within a few % can be achieved with better standards and calibration. Take care Sensitivities Si for peak to peak height of differentiated Auger peak different from the one for original Auger peak(with background subtraction) ...
... Error in AES: analysis: < 15%, Error within a few % can be achieved with better standards and calibration. Take care Sensitivities Si for peak to peak height of differentiated Auger peak different from the one for original Auger peak(with background subtraction) ...
chapters 16-17 test re
... Remember to show your work as well as units. You can use one 3x5 card (front and back) on the test as notes. The only thing I will give you will be a Periodic Table. Questions #1-10 are True or False. Write True or False on the blank next to each question. 1. _______ A chemical reaction rate is defi ...
... Remember to show your work as well as units. You can use one 3x5 card (front and back) on the test as notes. The only thing I will give you will be a Periodic Table. Questions #1-10 are True or False. Write True or False on the blank next to each question. 1. _______ A chemical reaction rate is defi ...
Toluenediamine
... The reduction of dinitrotoluene is characterized by its strong exotherm of >1100 kJ/mol. Due to this fact combined with the thermal instability of the dinitro compound certain processing requirements must be considered. Gas-phase reaction of dinitrotoluene can merely be realized and particular preca ...
... The reduction of dinitrotoluene is characterized by its strong exotherm of >1100 kJ/mol. Due to this fact combined with the thermal instability of the dinitro compound certain processing requirements must be considered. Gas-phase reaction of dinitrotoluene can merely be realized and particular preca ...
CHEMISTry is life - World of Teaching
... try every combination possible from the three chemicals that they are given. The reactions can be conducted in sealed plastic baggies. After making some initial observations, students must conduct an experiment of their choice based on the scientific method. ...
... try every combination possible from the three chemicals that they are given. The reactions can be conducted in sealed plastic baggies. After making some initial observations, students must conduct an experiment of their choice based on the scientific method. ...
Adv review key
... J) Draw the electron dot diagram (Lewis Dot Structure) and then tell if it would give up or take on electrons to get a full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
... J) Draw the electron dot diagram (Lewis Dot Structure) and then tell if it would give up or take on electrons to get a full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
APS 1st semester exam review 2016
... J) Draw the electron dot diagram (Lewis Dot Structure) and then tell if it would give up or take on electrons to get a full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
... J) Draw the electron dot diagram (Lewis Dot Structure) and then tell if it would give up or take on electrons to get a full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
Assignment Chemistry Class XI (2016-17)
... 15. If (P + a/V2) (V – b)=RT, where the symbols have their usual meanings, then (a/b) has a dimension of………. 16. Force(F) and density(d) are related as F = α/ (β+√d) (i)then the dimensions of α are………(ii) then the dimensions of β are…….. 17. In an experiment refractive index of glass was observed to ...
... 15. If (P + a/V2) (V – b)=RT, where the symbols have their usual meanings, then (a/b) has a dimension of………. 16. Force(F) and density(d) are related as F = α/ (β+√d) (i)then the dimensions of α are………(ii) then the dimensions of β are…….. 17. In an experiment refractive index of glass was observed to ...
Chapter 1: Fundamental Concepts
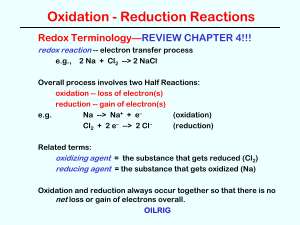
... one species is oxidized and one is reduced. The two processes will always occur together. • When has a redox reaction occurred? – If there is a change in the oxidation state of any element in the reaction, a redox reaction has happened. – Remember that if something is oxidized, something else must b ...
... one species is oxidized and one is reduced. The two processes will always occur together. • When has a redox reaction occurred? – If there is a change in the oxidation state of any element in the reaction, a redox reaction has happened. – Remember that if something is oxidized, something else must b ...
PPT - kimscience.com
... WORK SPONTANEOUSLY? • When you connect 2 metals from different positions on the activity series - it creates a electrical potential energy difference b/w the metals. • The greater the activity b/w the two metals, the greater the electrical potential ...
... WORK SPONTANEOUSLY? • When you connect 2 metals from different positions on the activity series - it creates a electrical potential energy difference b/w the metals. • The greater the activity b/w the two metals, the greater the electrical potential ...
Photoredox catalysis
_Schematic.png?width=300)
Photoredox catalysis is a branch of catalysis that harnesses the energy of visible light to accelerate a chemical reaction via a single-electron transfer. This area is named as a combination of ""photo-"" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that otherwise would not react. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes and semiconductors. While each class of materials has advantages, soluble transition-metal complexes are used most often.Study of this branch of catalysis led to the development of new methods to accomplish known and new chemical transformations. One attraction to the area is that photoredox catalysts are often less toxic than other reagents often used to generate free radicals, such as organotin reagents. Furthermore, while photoredox catalysts generate potent redox agents while exposed to light, they are innocuous under ordinary conditions Thus transition-metal complex photoredox catalysts are in some ways more attractive than stoichiometric redox agents such as quinones. The properties of photoredox catalysts can be modified by changing ligands and the metal, reflecting the somewhat modular nature of the catalyst.While photoredox catalysis has most often been applied to generate known reactive intermediates in a novel way, the study of this mode of catalysis led to the discovery of new organic reactions, such as the first direct functionalization of the β-arylation of saturated aldehydes. Although the D3-symmetric transition-metal complexes used in many photoredox-catalyzed reactions are chiral, the use of enantioenriched photoredox catalysts led to low levels of enantioselectivity in a photoredox-catalyzed aryl-aryl coupling reaction, suggesting that the chiral nature of these catalysts is not yet a highly effective means of transmitting stereochemical information in photoredox reactions. However, while synthetically useful levels of enantioselectivity have not been achieved using chiral photoredox catalysts alone, optically-active products have been obtained through the synergistic combination of photoredox catalysis with chiral organocatalysts such as secondary amines and Brønsted acids.