2015 Dr. Jay L. Wile, All rights reserved.
... oxygen to make 49.9 grams of a gas, along with some leftover oxygen. Are the two gases he made the same? ...
... oxygen to make 49.9 grams of a gas, along with some leftover oxygen. Are the two gases he made the same? ...
This article was published in an Elsevier journal. The attached copy
... an easy phase separation. In the subsequent step, Section 3 including reactions (9) and (10), the separation of HI from L − 2, the heavier iodine/iodide–water phase, is the most critical scenario of the cycle [4] and believed to be the most expensive and energy-consuming step [5]. After establishing ...
... an easy phase separation. In the subsequent step, Section 3 including reactions (9) and (10), the separation of HI from L − 2, the heavier iodine/iodide–water phase, is the most critical scenario of the cycle [4] and believed to be the most expensive and energy-consuming step [5]. After establishing ...
Stoichiometry - HCC Learning Web
... calculate the moles of each to determine which is the limiting reactant. Step 3: Calculate the moles of "desired" substance from your answer in Step 2 using the coefficients from the balanced chemical equation. If more than one reactant was given originally, you can calculate the moles of product tw ...
... calculate the moles of each to determine which is the limiting reactant. Step 3: Calculate the moles of "desired" substance from your answer in Step 2 using the coefficients from the balanced chemical equation. If more than one reactant was given originally, you can calculate the moles of product tw ...
Chemistry - cloudfront.net
... 43. given a covalent bond between two atoms, be able to discern if the bond is polar or non-polar 44. given a molecular formula, be able to draw the Lewis structure to be able to tell if the molecule is polar or non-polar 45. know what an induction arrow signifies (points to the more electronegative ...
... 43. given a covalent bond between two atoms, be able to discern if the bond is polar or non-polar 44. given a molecular formula, be able to draw the Lewis structure to be able to tell if the molecule is polar or non-polar 45. know what an induction arrow signifies (points to the more electronegative ...
Energy Matters - Perth Grammar
... An electron diagram to show the overlap of electron clouds in a water molecule is shown left. Draw a diagram to show electrons in the outer shells in the following molecules. ...
... An electron diagram to show the overlap of electron clouds in a water molecule is shown left. Draw a diagram to show electrons in the outer shells in the following molecules. ...
Chapter 20 - public.asu.edu
... Eoox = 0.76 V since Eored = 0.00 V Reference to Hydrogen Electrode The E of the hydrogen electrode is defined as 0.00 V. What would be the values of Eo for other half-reactions if this were defined as 1.00 V? The Eo of the hydrogen electrode is defined as 0.00 V. What would be the values of Eo for o ...
... Eoox = 0.76 V since Eored = 0.00 V Reference to Hydrogen Electrode The E of the hydrogen electrode is defined as 0.00 V. What would be the values of Eo for other half-reactions if this were defined as 1.00 V? The Eo of the hydrogen electrode is defined as 0.00 V. What would be the values of Eo for o ...
ChemConnections
... = ( 8 mol x 256.66 J/mol K) - [(1 mol x 430.211 J/mol K) + (12 mol x 205.0 J/mol K)] = (2,053.28 J/K) - [(430.211 J/K) + (2,460.0 J/K)] = 2,053.28 J/K - 2,890.211 J/K = - 836.931 J/K ...
... = ( 8 mol x 256.66 J/mol K) - [(1 mol x 430.211 J/mol K) + (12 mol x 205.0 J/mol K)] = (2,053.28 J/K) - [(430.211 J/K) + (2,460.0 J/K)] = 2,053.28 J/K - 2,890.211 J/K = - 836.931 J/K ...
Standard enthalpy of formation
... formation for each of the steps involved in the process. This is especially useful for very long reactions with many intermediate steps and compounds. Chemists may use standard enthalpies of formation for a reaction that is hypothetical. For instance carbon and hydrogen will not directly react to fo ...
... formation for each of the steps involved in the process. This is especially useful for very long reactions with many intermediate steps and compounds. Chemists may use standard enthalpies of formation for a reaction that is hypothetical. For instance carbon and hydrogen will not directly react to fo ...
Chemistry 180-213B Introductory Physical
... mechanical equivalent of heat. He introduced what came to be known as Carnot’s cycle, and established Carnot’s principle. Carnot’s book had been overlooked until B. P. E. Clapeyron (1834) gave Carnot’s theory an analytical and graphical expression by making use of the indicator diagram devised by Wa ...
... mechanical equivalent of heat. He introduced what came to be known as Carnot’s cycle, and established Carnot’s principle. Carnot’s book had been overlooked until B. P. E. Clapeyron (1834) gave Carnot’s theory an analytical and graphical expression by making use of the indicator diagram devised by Wa ...
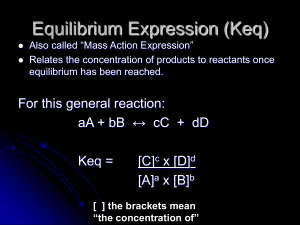
Equilibrium Expression (Keq)
... values (Molarity) into the Keq expression you will get a specific number (The is a unitless number and is unique to that reaction.) The only thing that can change the value of Keq is a change in temperature. ...
... values (Molarity) into the Keq expression you will get a specific number (The is a unitless number and is unique to that reaction.) The only thing that can change the value of Keq is a change in temperature. ...
Redox Reactions and Electrochemistry
... balanced. The next step is to combine the two halfreactions to form an overall equation. 6) Multiply through each half-reactions by appropriate coefficients to match electrons in each half-reaction. (i.e. number of electrons lost by the oxidized species must equal the number gained by the reduced on ...
... balanced. The next step is to combine the two halfreactions to form an overall equation. 6) Multiply through each half-reactions by appropriate coefficients to match electrons in each half-reaction. (i.e. number of electrons lost by the oxidized species must equal the number gained by the reduced on ...
Writing Net Ionic Equations
... formed in acid-base neutralization reactions. Acetic acid is an example of an acid that is primarily molecular (weak electrolyte) when placed in water. Reversible Reactions If a double replacement reaction does not go to completion (no precipitate, gas or molecular species is formed), then the react ...
... formed in acid-base neutralization reactions. Acetic acid is an example of an acid that is primarily molecular (weak electrolyte) when placed in water. Reversible Reactions If a double replacement reaction does not go to completion (no precipitate, gas or molecular species is formed), then the react ...
Final Exam Review
... c. Definite volume; shape of container; no intermolecular attractions d. Volume and shape of container; no intermolecular attractions e. Volume and shape of container; strong intermolecular attractions 102. Which transformation is evaporation? a. liquid ---> solid d. solid ---> gas b. liquid ---> ga ...
... c. Definite volume; shape of container; no intermolecular attractions d. Volume and shape of container; no intermolecular attractions e. Volume and shape of container; strong intermolecular attractions 102. Which transformation is evaporation? a. liquid ---> solid d. solid ---> gas b. liquid ---> ga ...
chemistry (9189)
... Candidates will be required to answer a total of six questions, two from Section A one from Section B, two from Section C, plus any other. ...
... Candidates will be required to answer a total of six questions, two from Section A one from Section B, two from Section C, plus any other. ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.