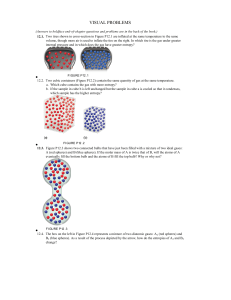
Pauling Scale of Electronegativities for the Various Elements
... and Ba2+. Metal sulfides are the least soluble followed by H2S; hydroxides are only slightly more soluble than sulfides. ...
... and Ba2+. Metal sulfides are the least soluble followed by H2S; hydroxides are only slightly more soluble than sulfides. ...
Ch16 - WordPress.com
... is 48.8 at 455°C. An equilibrium mixture in a 2.0 L vessel at this temperature contains 0.220 mol of H2 and 0.110 mol of I2. a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0.44 mol of H2 and 0.44 ...
... is 48.8 at 455°C. An equilibrium mixture in a 2.0 L vessel at this temperature contains 0.220 mol of H2 and 0.110 mol of I2. a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0.44 mol of H2 and 0.44 ...
Chapter 16: Energy and Chemical Change
... of energy remains constant. Energy is conserved. To better understand the conservation of energy, suppose you have money in two accounts at a bank and you transfer funds from one account to the other. Although the amount of money in each account has changed, the total amount of your money in the ban ...
... of energy remains constant. Energy is conserved. To better understand the conservation of energy, suppose you have money in two accounts at a bank and you transfer funds from one account to the other. Although the amount of money in each account has changed, the total amount of your money in the ban ...
1. Which idea of John Dalton is no longer considered part of the
... containing N 2, O2 and CO2 is 30 atm. If the partial pressure of N2 is 4 atm, and the partial pressure of O2 is 6 atm, what is the partial pressure of CO2? ...
... containing N 2, O2 and CO2 is 30 atm. If the partial pressure of N2 is 4 atm, and the partial pressure of O2 is 6 atm, what is the partial pressure of CO2? ...
Chapter 16: Reaction Rates
... Objectives ◗ Calculate average rates of chemical reactions from experimental data. ◗ Relate rates of chemical reactions to collisions between reacting particles. ...
... Objectives ◗ Calculate average rates of chemical reactions from experimental data. ◗ Relate rates of chemical reactions to collisions between reacting particles. ...
aq - HCC Learning Web
... • Aqueous solutions of lead(II) nitrate and potassium iodide produce a yellow precipitate of lead(II) iodide and an aqueous solution of potassium nitrate Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) • Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calciu ...
... • Aqueous solutions of lead(II) nitrate and potassium iodide produce a yellow precipitate of lead(II) iodide and an aqueous solution of potassium nitrate Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) • Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calciu ...
Pre-AP Chemistry Final Exam Review 1. Write the name for
... □Know that single replacement reactions will use either the metals activity series or the halogen activity series to predict if the reaction will take place. □Know how to balance the equation by adding coefficients until the numbers of atoms are equal on each side. □Know how to properly use subscrip ...
... □Know that single replacement reactions will use either the metals activity series or the halogen activity series to predict if the reaction will take place. □Know how to balance the equation by adding coefficients until the numbers of atoms are equal on each side. □Know how to properly use subscrip ...
Relation between the characteristic molecular volume and
... (f = G, H, S) are greatly influenced by the hydrophobic effect. Their experimental values are a good starting point to analyze this phenomenon using various models of hydration. Alternatively, one can study hydration of idealized objects such as hard particles in computer simulations. The energy of h ...
... (f = G, H, S) are greatly influenced by the hydrophobic effect. Their experimental values are a good starting point to analyze this phenomenon using various models of hydration. Alternatively, one can study hydration of idealized objects such as hard particles in computer simulations. The energy of h ...
anna-chrobok-silesian-university-of-technology
... - Diels-Alder reaction, - oxidation of alcohols and ketones. IONIC LIQUIDS as homogeneous and heterogeneous catalysts Recycling of ionic liquids prevents them from: - ending up in the aquatic environment, - release into the atmosphere (low volatility). ...
... - Diels-Alder reaction, - oxidation of alcohols and ketones. IONIC LIQUIDS as homogeneous and heterogeneous catalysts Recycling of ionic liquids prevents them from: - ending up in the aquatic environment, - release into the atmosphere (low volatility). ...
Standard Half Cell Potentials
... and a pressure of 1 bar. Pure substances (solids, liquids and gases) should be in their normal state at this temperature and pressure. Solutions should have an activity* equal to one, which we will approximate here as a concentration equal to 1 mol L-1 The potential of a cell under standard conditio ...
... and a pressure of 1 bar. Pure substances (solids, liquids and gases) should be in their normal state at this temperature and pressure. Solutions should have an activity* equal to one, which we will approximate here as a concentration equal to 1 mol L-1 The potential of a cell under standard conditio ...
Intermolecular Forces
... Casimir and Polder3 also showed that retardation effects weaken the dispersion force at separations of the order of the wavelength of the electronic absorption bands of the interacting molecules, which is typically lop7m. The retarded dispersion energy varies as K7 at large R and is determined by th ...
... Casimir and Polder3 also showed that retardation effects weaken the dispersion force at separations of the order of the wavelength of the electronic absorption bands of the interacting molecules, which is typically lop7m. The retarded dispersion energy varies as K7 at large R and is determined by th ...
Introduction Statistical Thermodynamics
... However, it is most of the times much better to think and to carefully select an appropriate ensemble. For this it is important to know how to simulate in the various ...
... However, it is most of the times much better to think and to carefully select an appropriate ensemble. For this it is important to know how to simulate in the various ...
Unit 10: Chemical Reactions
... The substances that undergo a chemical reaction are the reactants. The new substances formed are the products. Special symbols are written after formulas in equations to show a substance’s state. The designations for solid, liquid, or gas, are (s), (l), and (g), respectively. A substance dissolv ...
... The substances that undergo a chemical reaction are the reactants. The new substances formed are the products. Special symbols are written after formulas in equations to show a substance’s state. The designations for solid, liquid, or gas, are (s), (l), and (g), respectively. A substance dissolv ...
2013-2014
... Using zinc dust instead of chunks. Using 200 cm3 of 1.0 M HCl in place of 100 cm3. Using 2.0 M HCl instead of 1.0 M HCl. ...
... Using zinc dust instead of chunks. Using 200 cm3 of 1.0 M HCl in place of 100 cm3. Using 2.0 M HCl instead of 1.0 M HCl. ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.