Nickel catalyst auto-reduction during steam reforming of bio
... Although the reduction had stopped, the calculated reduction rate did not return to zero (Fig. 4a) and consequently the calculated conversion was larger than 100% (Fig. 4b, kinetics model will be discussed later). This error was possibly caused by propagation of experimental error in the calculatio ...
... Although the reduction had stopped, the calculated reduction rate did not return to zero (Fig. 4a) and consequently the calculated conversion was larger than 100% (Fig. 4b, kinetics model will be discussed later). This error was possibly caused by propagation of experimental error in the calculatio ...
Review Unit: Chemistry Review
... part to our understanding and application of chemistry. Some chemicals are harmful to people or the environment, but many are integral to life, such as the carbon dioxide, oxygen, water, and glucose in the cycle of photosynthesis and cellular respiration. The air we breathe and the food we eat are a ...
... part to our understanding and application of chemistry. Some chemicals are harmful to people or the environment, but many are integral to life, such as the carbon dioxide, oxygen, water, and glucose in the cycle of photosynthesis and cellular respiration. The air we breathe and the food we eat are a ...
03-Chemical Rxns n Stoichiometry
... to establish identities of products via further research, etc. When identities of reactants and products are established, we can write their formulas. Still, the number of atoms of each element must be the same on both sides of the equation to adhere to the Laws of Conservation of Matter and Mass. T ...
... to establish identities of products via further research, etc. When identities of reactants and products are established, we can write their formulas. Still, the number of atoms of each element must be the same on both sides of the equation to adhere to the Laws of Conservation of Matter and Mass. T ...
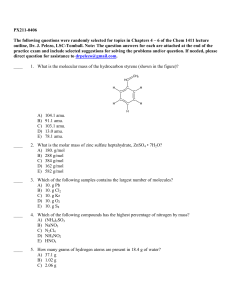
PX211-0406 The following questions were randomly selected for
... 29. Which of the following statements is least likely to be true of a sample of nitrogen gas at STP? A) Collisions between the gaseous molecules are elastic. B) The intermolecular forces between nitrogen molecules are not negligible. C) Molecules of gaseous nitrogen are in constant random motion. D) ...
... 29. Which of the following statements is least likely to be true of a sample of nitrogen gas at STP? A) Collisions between the gaseous molecules are elastic. B) The intermolecular forces between nitrogen molecules are not negligible. C) Molecules of gaseous nitrogen are in constant random motion. D) ...
Word - icho39.chem.msu.ru
... If the oxygen pressure is above the equilibrium value, the reaction will proceed from the left to the right to reach the equilibrium state. So the answer is 0.0825 Torr < p(O2) < 1.00 Torr. 2. The reaction proceeds forward as long as G, not G is negative! The following equation is valid for the r ...
... If the oxygen pressure is above the equilibrium value, the reaction will proceed from the left to the right to reach the equilibrium state. So the answer is 0.0825 Torr < p(O2) < 1.00 Torr. 2. The reaction proceeds forward as long as G, not G is negative! The following equation is valid for the r ...
Part One: Ions in Aqueous Solution A. Electrolytes and Non
... Titration = process in which a solution of one reactant (the titrant) is carefully added to a solution of another reactant. Volume of titrant required for complete reaction is measured. ...
... Titration = process in which a solution of one reactant (the titrant) is carefully added to a solution of another reactant. Volume of titrant required for complete reaction is measured. ...
Chapter 4-5
... Aqueous reactions Aqueous reactions can be grouped into three general categories; a. precipitation, b. acid-base and c. Oxidation reactions – Reactions are driven from reactants to products by some energetic force that pushes them along. 1. Precipitation Reactions • Driving force = removal of mater ...
... Aqueous reactions Aqueous reactions can be grouped into three general categories; a. precipitation, b. acid-base and c. Oxidation reactions – Reactions are driven from reactants to products by some energetic force that pushes them along. 1. Precipitation Reactions • Driving force = removal of mater ...
Stoichiometry
... If you start with 6 eggs, how many cupcakes would you make? If you started with 4 cups of flour, how many cakes could you make? If you made 6 cupcakes, how many tubs of frosting did you need? If you started with 1 ½ cups of flour, how many cupcakes could you make? ...
... If you start with 6 eggs, how many cupcakes would you make? If you started with 4 cups of flour, how many cakes could you make? If you made 6 cupcakes, how many tubs of frosting did you need? If you started with 1 ½ cups of flour, how many cupcakes could you make? ...
105
... The oxidation number of nitrogen increases from −3 to +4, an increase of 7. The oxidation number of oxygen decreases from 0 to −2, a decrease of 2. The least common multiple of 7 and 2 is 14. In this case, two nitrogen atoms must react for every seven oxygen atoms so that the total increase and decr ...
... The oxidation number of nitrogen increases from −3 to +4, an increase of 7. The oxidation number of oxygen decreases from 0 to −2, a decrease of 2. The least common multiple of 7 and 2 is 14. In this case, two nitrogen atoms must react for every seven oxygen atoms so that the total increase and decr ...
full text pdf
... provided a product with a narrow particle size distribution. The 160 UPZ mill from Hosokawa-Alpine was used for impact milling. Milling of the material was due to impact of the steel tines spaced on the shield and on the spinning rotor. The milling was conducted at rotor speed of 11 400 rpm and with ...
... provided a product with a narrow particle size distribution. The 160 UPZ mill from Hosokawa-Alpine was used for impact milling. Milling of the material was due to impact of the steel tines spaced on the shield and on the spinning rotor. The milling was conducted at rotor speed of 11 400 rpm and with ...
AP Chemistry Lab Manual
... as there will often times be a quiz over that content. If there are questions you are supposed to answer, do them on a separate sheet of paper and hand them in as your ticket into lab. If there is a code word in the procedure or weird instructions be prepared to follow them. 2. Some labs will be ful ...
... as there will often times be a quiz over that content. If there are questions you are supposed to answer, do them on a separate sheet of paper and hand them in as your ticket into lab. If there is a code word in the procedure or weird instructions be prepared to follow them. 2. Some labs will be ful ...
File
... as there will often times be a quiz over that content. If there are questions you are supposed to answer, do them on a separate sheet of paper and hand them in as your ticket into lab. If there is a code word in the procedure or weird instructions be prepared to follow them. 2. Some labs will be ful ...
... as there will often times be a quiz over that content. If there are questions you are supposed to answer, do them on a separate sheet of paper and hand them in as your ticket into lab. If there is a code word in the procedure or weird instructions be prepared to follow them. 2. Some labs will be ful ...
Extended Lagrangian free energy molecular dynamics
... = {Ri },8, 9 under the constraints of correct electron occupation, Tr[D] = Nocc . The electronic temperature Te can be different from the ionic temperature Tion . We assume that U[R; D] is the total electronic energy, including nuclear ion-ion repulsions, in self-consistent density functional theory ...
... = {Ri },8, 9 under the constraints of correct electron occupation, Tr[D] = Nocc . The electronic temperature Te can be different from the ionic temperature Tion . We assume that U[R; D] is the total electronic energy, including nuclear ion-ion repulsions, in self-consistent density functional theory ...
Higher Chemistry Specimen Question Paper
... 18. Which of the following elements is the strongest reducing agent? A Lithium B Bromine C Fluorine D Aluminium 19. 45 cm3 of a solution could be most accurately measured out using a A ...
... 18. Which of the following elements is the strongest reducing agent? A Lithium B Bromine C Fluorine D Aluminium 19. 45 cm3 of a solution could be most accurately measured out using a A ...
Influence of Storage on the Composition of Clarified Apple Juice
... to 5, hydrolysis appeared to be the major cause of sucrose reduction (and reducing sugars increase) at a rate determined by pH and temperature. Akhavan and Wrolstad (1980) verified that slight losses (6%) in total sugars occur after 112 days of storage at 37°C of pear concentrate. Stadtman (1948) co ...
... to 5, hydrolysis appeared to be the major cause of sucrose reduction (and reducing sugars increase) at a rate determined by pH and temperature. Akhavan and Wrolstad (1980) verified that slight losses (6%) in total sugars occur after 112 days of storage at 37°C of pear concentrate. Stadtman (1948) co ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.