
Tro Chemistry a Molecular Approach, 3E
... R>NA = 1.38 * 10 - 23 J>K) and W is the number of energetically equivalent ways to arrange the components of the system. Since W is unitless (it is simply a number), the units of entropy are joules per kelvin (J>K). We talk about the significance of the units shortly. As you can see from the equatio ...
... R>NA = 1.38 * 10 - 23 J>K) and W is the number of energetically equivalent ways to arrange the components of the system. Since W is unitless (it is simply a number), the units of entropy are joules per kelvin (J>K). We talk about the significance of the units shortly. As you can see from the equatio ...
© NCERT not to be republished
... (ii) Both assertion and reason are true but reason is not the correct explanation of assertion. ...
... (ii) Both assertion and reason are true but reason is not the correct explanation of assertion. ...
Exam - Vcaa
... The equation for the redox reaction is 2CuO(s) 2Cu(s) + O2(g) The gas passing through the tube prevented the copper from re-oxidising to CuO. The students weighed: • the empty tube • the tube and CuO before heating • the tube and Cu after heating and cooling. They found that the percentage by mass ...
... The equation for the redox reaction is 2CuO(s) 2Cu(s) + O2(g) The gas passing through the tube prevented the copper from re-oxidising to CuO. The students weighed: • the empty tube • the tube and CuO before heating • the tube and Cu after heating and cooling. They found that the percentage by mass ...
One-Pot Catalytic Conversion of Cellulose and of Woody
... from Aldrich (#C6288). The Cu20-PMO catalyst typically used has a 15/60/25 Cu/Mg/Al molar ratio and was prepared by calcining a copper-doped hydrotalcite as previously reported.12,13 Small-Scale Batch Reactions. In a typical run, a finely divided substrate (wood or cellulose), catalyst (usually Cu20 ...
... from Aldrich (#C6288). The Cu20-PMO catalyst typically used has a 15/60/25 Cu/Mg/Al molar ratio and was prepared by calcining a copper-doped hydrotalcite as previously reported.12,13 Small-Scale Batch Reactions. In a typical run, a finely divided substrate (wood or cellulose), catalyst (usually Cu20 ...
Плеханов В
... Covalent bond contribution is significantly great even at great difference in atoms electronegativities. Isotope species behavour laws can be explained in terms of electron properties, electron molecular structures, reaction products and its interactions with the environment. A great number of exper ...
... Covalent bond contribution is significantly great even at great difference in atoms electronegativities. Isotope species behavour laws can be explained in terms of electron properties, electron molecular structures, reaction products and its interactions with the environment. A great number of exper ...
Fall 2002 Honors
... 15. (5 pts) Nitrogen and phosphorus are in the same group, so you would expect them to exhibit similar chemical properties. NCl3, PCl3, and PCl5 are all stable compounds that are easily synthesized in the lab. However, NCl5 has never been synthesized or observed. Why would phosphorus form two compou ...
... 15. (5 pts) Nitrogen and phosphorus are in the same group, so you would expect them to exhibit similar chemical properties. NCl3, PCl3, and PCl5 are all stable compounds that are easily synthesized in the lab. However, NCl5 has never been synthesized or observed. Why would phosphorus form two compou ...
Aggregation and Adsorption at Interfaces
... presence of hydrocarbon groups in dissolved amphiphilic molecules causes distortion of this solvent structure apparently increasing the free energy of the system. This is known as the hydrophobic effect [1]. Less work is required to bring a surfactant molecule to the surface than a water molecule, s ...
... presence of hydrocarbon groups in dissolved amphiphilic molecules causes distortion of this solvent structure apparently increasing the free energy of the system. This is known as the hydrophobic effect [1]. Less work is required to bring a surfactant molecule to the surface than a water molecule, s ...
TERMS AND DEFINITIONS IN THERMOCHEMISTRY
... This is the law on which the whole of thermochemistry is based. It states that the enthalpy change for a process does not depend on the nature of any intermediate steps involved in bringing the process about. That is, the enthalpy change does not depend on the path chosen to accomplish the process, ...
... This is the law on which the whole of thermochemistry is based. It states that the enthalpy change for a process does not depend on the nature of any intermediate steps involved in bringing the process about. That is, the enthalpy change does not depend on the path chosen to accomplish the process, ...
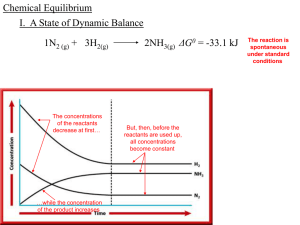
(K c ) [A] - Knockhardy
... This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purp ...
... This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purp ...
PHYSICAL SETTING CHEMISTRY
... 69 What is the evidence that the average kinetic energy of the particles of this substance is increasing during the first three minutes? [1] 70 The heat of fusion for this substance is 122 joules per gram. How many joules of heat are needed to melt 7.50 grams of this substance at its melting point? ...
... 69 What is the evidence that the average kinetic energy of the particles of this substance is increasing during the first three minutes? [1] 70 The heat of fusion for this substance is 122 joules per gram. How many joules of heat are needed to melt 7.50 grams of this substance at its melting point? ...
2014 International Practice Exam: Chemistry
... SECTION II: Free Response After .the .break, .say: May I have everyone’s attention? Place your Student Pack on your desk. . . . ...
... SECTION II: Free Response After .the .break, .say: May I have everyone’s attention? Place your Student Pack on your desk. . . . ...
Mnemonic Devices - Free WonderKids-e
... 4) Many direct combination reactions are combustion reactions, but not all combustion reactions are necessarily direct combination reactions because there are two reactants that combine, but more than one product is formed. Consider the combustion reaction in which gasohol burns in a car’s engine. G ...
... 4) Many direct combination reactions are combustion reactions, but not all combustion reactions are necessarily direct combination reactions because there are two reactants that combine, but more than one product is formed. Consider the combustion reaction in which gasohol burns in a car’s engine. G ...
towards the synthesis of functionalised macrocyclic receptors
... 84, is described. Both macrocycles were fully characterised using elemental analysis, 1H NMR, 13C NMR, and mass spectroscopy. The solid-state structure of 82 was also determined using X-ray crystallography. During these investigations it was shown that Cs+ can be replaced by K+ as an effective templ ...
... 84, is described. Both macrocycles were fully characterised using elemental analysis, 1H NMR, 13C NMR, and mass spectroscopy. The solid-state structure of 82 was also determined using X-ray crystallography. During these investigations it was shown that Cs+ can be replaced by K+ as an effective templ ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.










![(K c ) [A] - Knockhardy](http://s1.studyres.com/store/data/011755527_1-914ea907d1ff7656ef398ad87316c94c-300x300.png)











