7.1 CHEMICAL SYSTEMS IN EQUILIBRIUM: Dynamic Equilibrium in
... In 1884 the French chemist and engineer Henry-Louis Le Chatelier proposed one of the central concepts of chemical equilibrium. Le Chatelier's principle can be stated as follows: A change in one of the variables that describe a system at equilibrium produces a shift in the position of the equilibrium ...
... In 1884 the French chemist and engineer Henry-Louis Le Chatelier proposed one of the central concepts of chemical equilibrium. Le Chatelier's principle can be stated as follows: A change in one of the variables that describe a system at equilibrium produces a shift in the position of the equilibrium ...
Book-Abstracts - The Fritz Haber Center for Molecular dynamics
... Schrodinger equation for a period of 30 femtoseconds. As a function of incident kinetic energy, the dissociation yield follows the experimental trend. An attempt at simulation employing only the lowest adiabatic surface failed, qualitatively disagreeing with both experiment and non-adiabatic calcula ...
... Schrodinger equation for a period of 30 femtoseconds. As a function of incident kinetic energy, the dissociation yield follows the experimental trend. An attempt at simulation employing only the lowest adiabatic surface failed, qualitatively disagreeing with both experiment and non-adiabatic calcula ...
Thermodynamic course year 99-00
... respectively. Calculate the work w for: A. abrupt transitions, by weight exchange to states 2 and 3 in sequence. B. Abrupt transition directly to state 3. C. reversible transition to state 3. D. abrupt transition from 3 to 1. ...
... respectively. Calculate the work w for: A. abrupt transitions, by weight exchange to states 2 and 3 in sequence. B. Abrupt transition directly to state 3. C. reversible transition to state 3. D. abrupt transition from 3 to 1. ...
Topic 4
... We obtained the same conclusion that NH3 is a base using this theory; however, we didn’t look at the production of OH- to make the decision. It was based on NH3 accepting a proton in the aqueous rxn. ...
... We obtained the same conclusion that NH3 is a base using this theory; however, we didn’t look at the production of OH- to make the decision. It was based on NH3 accepting a proton in the aqueous rxn. ...
TRY THIS
... C) Systems, Work and Heat 1) System: …the region in which we are interested: such as a flask of gas, a beaker of acid, a reaction mixture, or a muscle fiber (Atkins. p. 236). Everything else, such as a water bath, in which a reaction is immersed (in another beaker) is referred to as the surrounding ...
... C) Systems, Work and Heat 1) System: …the region in which we are interested: such as a flask of gas, a beaker of acid, a reaction mixture, or a muscle fiber (Atkins. p. 236). Everything else, such as a water bath, in which a reaction is immersed (in another beaker) is referred to as the surrounding ...
0922085
... This document contains the lists of questions proposed by CCNR in respect of knowledge of physics and chemistry for the “chemicals” examination: ...
... This document contains the lists of questions proposed by CCNR in respect of knowledge of physics and chemistry for the “chemicals” examination: ...
equilibrium theory of the kaolinite
... finally hydrate on kaolinite at relative pressures near one (Martin, 1959; Jurinak, 1963) is no contradiction of the theory, because the exchangeable cations on a wet clay interact directly with the mineral surface hydration water rather than with water vapor. For such an interaction, equation (2) m ...
... finally hydrate on kaolinite at relative pressures near one (Martin, 1959; Jurinak, 1963) is no contradiction of the theory, because the exchangeable cations on a wet clay interact directly with the mineral surface hydration water rather than with water vapor. For such an interaction, equation (2) m ...
The applicability of activities in kinetic expressions Haubrock, J.
... In this study absorption rate experiments have been carried out in the pseudo-first-order absorption rate regime. The experiments have been interpreted using a new activity based kinetic rate expression instead of the traditional concentration-based rate expression. A series of CO2 absorption experim ...
... In this study absorption rate experiments have been carried out in the pseudo-first-order absorption rate regime. The experiments have been interpreted using a new activity based kinetic rate expression instead of the traditional concentration-based rate expression. A series of CO2 absorption experim ...
Unit 5 Test Review
... produced if a given amount of moles of reactant was reacted. Which quantities would be essential in order to solve such a problem? Bubble in all that apply - but only those that are essential to this calculation. a. The molar mass of the reactant b. The molar mass of the product c. The coefficients ...
... produced if a given amount of moles of reactant was reacted. Which quantities would be essential in order to solve such a problem? Bubble in all that apply - but only those that are essential to this calculation. a. The molar mass of the reactant b. The molar mass of the product c. The coefficients ...
chemistry advanced may 2010 marking scheme
... (i) State and explain briefly the acid–base properties (if any) of the substances. CH4 is neutral since it does not accept or donate protons. (0.5) NH3 is a base since it accepts protons to form NH4+. (0.5) H2S is an acid donating a proton to form HS- (0.5); the HS- ion is a very weak acid and donat ...
... (i) State and explain briefly the acid–base properties (if any) of the substances. CH4 is neutral since it does not accept or donate protons. (0.5) NH3 is a base since it accepts protons to form NH4+. (0.5) H2S is an acid donating a proton to form HS- (0.5); the HS- ion is a very weak acid and donat ...
all practice examples
... A single step reversible reaction has an activation energy for the forward reaction (Ea,f) of 28.9 kJ and 41.8 kJ for the reverse reaction (Ea,r) Draw a potential energy level diagram and indicate H ...
... A single step reversible reaction has an activation energy for the forward reaction (Ea,f) of 28.9 kJ and 41.8 kJ for the reverse reaction (Ea,r) Draw a potential energy level diagram and indicate H ...
CHEMICAL REACTIONS AND CHEMICAL EQUATIONS
... Stoichiometry is one of the most important topics in chemistry. It involves the use of the chemical formulas , mole calculations, and chemical equations. Stoichiometry is also essential in industry , there, it is used to do cost and analysis for manufacturing chemicals. In fact, manufacturing proces ...
... Stoichiometry is one of the most important topics in chemistry. It involves the use of the chemical formulas , mole calculations, and chemical equations. Stoichiometry is also essential in industry , there, it is used to do cost and analysis for manufacturing chemicals. In fact, manufacturing proces ...
Title Variable anisotropy of ionic conduction in lithium nitride: Effect
... Department of Materials Science and Engineering, Kyoto University, Sakyo, Kyoto 606-8501, Japan and Nanostructures Research Laboratory, Japan Fine Ceramics Center, Atsuta, Nagoya 456-8587, Japan 共Received 5 September 2008; revised manuscript received 5 June 2009; published 27 July 2009兲 The formatio ...
... Department of Materials Science and Engineering, Kyoto University, Sakyo, Kyoto 606-8501, Japan and Nanostructures Research Laboratory, Japan Fine Ceramics Center, Atsuta, Nagoya 456-8587, Japan 共Received 5 September 2008; revised manuscript received 5 June 2009; published 27 July 2009兲 The formatio ...
Standard C-1: The student will demonstrate an understanding of
... combinations of acids and bases are mixed in a coffee cup calorimeter. Temperature change is measured and enthalpy is calculated. The reactions are chosen so that subtracting the chemical equation for the second reaction from the first reaction will give the chemical equation for the third reaction ...
... combinations of acids and bases are mixed in a coffee cup calorimeter. Temperature change is measured and enthalpy is calculated. The reactions are chosen so that subtracting the chemical equation for the second reaction from the first reaction will give the chemical equation for the third reaction ...
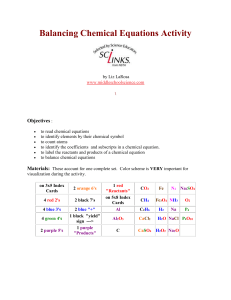
Balancing Chemical Equations Activity by Liz LaRosa www
... TEACHER NOTES: The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock inst ...
... TEACHER NOTES: The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock inst ...
Discover Chemical Changes - gk-12
... how they know it’s a chemical change and the clue that tipped them off to why it was a chemical change and not a physical change. After they’re finished, you should go through each of the stations to explain what the chemical change was and why it happened. Here are the chemical changes for the item ...
... how they know it’s a chemical change and the clue that tipped them off to why it was a chemical change and not a physical change. After they’re finished, you should go through each of the stations to explain what the chemical change was and why it happened. Here are the chemical changes for the item ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.