Document
... optical fibers for high-speed communications. We can produce polycrystalline ceramics that are highly transparent. The ability to make translucent and transparent polycrystalline ceramics was developed in the 1960s when it was discovered that small additions of MgO to Al 2O3 powder could produce a f ...
... optical fibers for high-speed communications. We can produce polycrystalline ceramics that are highly transparent. The ability to make translucent and transparent polycrystalline ceramics was developed in the 1960s when it was discovered that small additions of MgO to Al 2O3 powder could produce a f ...
File
... (group 17) atoms. The functional group for organic halides is the halogen atom. A functional group is a characteristic arrangement of atoms within a molecule that determines the most important chemical and physical properties of a class of compounds. Organic halides include many common products, suc ...
... (group 17) atoms. The functional group for organic halides is the halogen atom. A functional group is a characteristic arrangement of atoms within a molecule that determines the most important chemical and physical properties of a class of compounds. Organic halides include many common products, suc ...
IBM-finalrev - Madison Public Schools
... e. KBr _____________________ f. difficult to separate ________________ g. properties are different from the substances that form it ________________ h. formula stands for it ____________________ i. can be separated by hand _______________ ...
... e. KBr _____________________ f. difficult to separate ________________ g. properties are different from the substances that form it ________________ h. formula stands for it ____________________ i. can be separated by hand _______________ ...
flame test
... Purpose: To determine the energy of light released from atoms of certain metallic elements. Procedure: 1. Label each test tube with the chemical formulas of the compounds to be tested. 2. Get a scoopful of each compound and place in the appropriate test tube. 3. Get 5 popsicle sticks. 4. Fill beaker ...
... Purpose: To determine the energy of light released from atoms of certain metallic elements. Procedure: 1. Label each test tube with the chemical formulas of the compounds to be tested. 2. Get a scoopful of each compound and place in the appropriate test tube. 3. Get 5 popsicle sticks. 4. Fill beaker ...
DESCRIPTION FOR THE GENERAL PUBLIC Luminescent materials
... Luminescent materials are defined as the materials revealing light emission cause by external stimuli. There are several types of luminescence differing in the source of light generation. For instance, photoluminescence is induced by the absorption of photons, which means that the application of lig ...
... Luminescent materials are defined as the materials revealing light emission cause by external stimuli. There are several types of luminescence differing in the source of light generation. For instance, photoluminescence is induced by the absorption of photons, which means that the application of lig ...
Structure and Properties of Polymers
... Macromolecular Chemistry of the Academy of Sciences of the Czech Republic in Prague, one of the major institutes devoted to basic research in polymer science world-wide. From 1990 to 1998, he served two four-years terms as the director of the Institute. He is also professor of macromolecular chemist ...
... Macromolecular Chemistry of the Academy of Sciences of the Czech Republic in Prague, one of the major institutes devoted to basic research in polymer science world-wide. From 1990 to 1998, he served two four-years terms as the director of the Institute. He is also professor of macromolecular chemist ...
U2: Day 4 Notes
... What is light? o A form of electromagnetic radiation o Radiation carries energy through space o Diffracts like waves o Comes in packets – photoelectric effect – Einstein – particle o Use whichever one is “handy” o Theory: light is composed of photons that have both particle and wave properties o Lig ...
... What is light? o A form of electromagnetic radiation o Radiation carries energy through space o Diffracts like waves o Comes in packets – photoelectric effect – Einstein – particle o Use whichever one is “handy” o Theory: light is composed of photons that have both particle and wave properties o Lig ...
Grade 5
... c. Trace the development of the thermometer and the Fahrenheit and Celsius scales d. Analyze a thermogram of a house to determine where heat is being lost Light a. Students examine the special properties of light, nature, and behavior b. Observe light filtered through a prism-observing light colors ...
... c. Trace the development of the thermometer and the Fahrenheit and Celsius scales d. Analyze a thermogram of a house to determine where heat is being lost Light a. Students examine the special properties of light, nature, and behavior b. Observe light filtered through a prism-observing light colors ...
Homework 1
... C) visible light D) X-rays E) ultraviolet light A1: A Q2: If the wavelength of a light source suddenly doubles, what happens to its frequency? A) It is one half B) It doubles C) It is one quarter D) It is four times larger A2: A Q3: If light always behaved like particles and never like waves, it wou ...
... C) visible light D) X-rays E) ultraviolet light A1: A Q2: If the wavelength of a light source suddenly doubles, what happens to its frequency? A) It is one half B) It doubles C) It is one quarter D) It is four times larger A2: A Q3: If light always behaved like particles and never like waves, it wou ...
Prova de Inglês - redemat
... The optical behavior of a solid material is a function of its interactions with electromagnetic radiation having wavelengths within the visible region of the spectrum. Possible interactive phenomena include refraction, reflection, absorption, and transmission of incident light. Metals appear opaque ...
... The optical behavior of a solid material is a function of its interactions with electromagnetic radiation having wavelengths within the visible region of the spectrum. Possible interactive phenomena include refraction, reflection, absorption, and transmission of incident light. Metals appear opaque ...
test - Scioly.org
... A viewing screen is separated from a double-slit source by 1.2 meters. The distance between the two slids is 0.030 mm. The second-order bright fringe is 4.5 cm from the center line. 27. Determine the wavelength of the light. 28. Calculate the distance between adjacent bright fringes. ...
... A viewing screen is separated from a double-slit source by 1.2 meters. The distance between the two slids is 0.030 mm. The second-order bright fringe is 4.5 cm from the center line. 27. Determine the wavelength of the light. 28. Calculate the distance between adjacent bright fringes. ...
Chapter 2: Basic Optics
... Wavefronts are formed at right angles to the rays of light, and the light diminishes in proportion to the square of the distance. ...
... Wavefronts are formed at right angles to the rays of light, and the light diminishes in proportion to the square of the distance. ...
INTO THE LIGHT
... Todd McLellan and Thomas Kneubϋhler’s photographs capture moments that otherwise couldn’t be visible to the human eye. McLellan’s Disassembly series freezes disassembled vintage electronics in a moment of exploded flight. Kneubϋhler allows the bright lights of mountains electrified for night skiing ...
... Todd McLellan and Thomas Kneubϋhler’s photographs capture moments that otherwise couldn’t be visible to the human eye. McLellan’s Disassembly series freezes disassembled vintage electronics in a moment of exploded flight. Kneubϋhler allows the bright lights of mountains electrified for night skiing ...
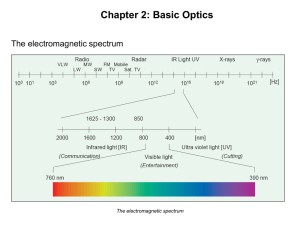
Electromagnetic Spectrum
... It can range from energy waves in the x-ray region all the way to the gamma ray region of the electromagnetic spectrum. Our human eye can only see the visible region of the electromagnetic spectrum. ...
... It can range from energy waves in the x-ray region all the way to the gamma ray region of the electromagnetic spectrum. Our human eye can only see the visible region of the electromagnetic spectrum. ...
Photopolymer
A photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing,.A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize in the presence of light either through internal or external initiation. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo curing is the formation of a thermoset network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene and oligomeric acrylates.Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet light is more energetic; however, the development of dye-based photoinitiator systems have allowed for the use of visible light, having potential advantages of processes that are more simple and safe to handle. UV curing in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization include high rates of polymerization and environmental benefits from elimination of volatile organic solvents.There are two general routes for photoinitiation: free radical and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.