How Fast Does Light Travel in Water vs. Air?
... universe is the speed of light in a vacuum (like outer space!), clocking in at a great 2.99 x 10 m/s. Light travels in waves, and we call this traveling propagation. Propagation of waves has both a speed and a direction, called the velocity. The velocity of light changes depends on the material it t ...
... universe is the speed of light in a vacuum (like outer space!), clocking in at a great 2.99 x 10 m/s. Light travels in waves, and we call this traveling propagation. Propagation of waves has both a speed and a direction, called the velocity. The velocity of light changes depends on the material it t ...
Wave Model
... A.) Spectroscopy: A method of analysis based on the interaction, absorption or production of light by matter. (also may include the interaction of electrons, ions or acoustics with matter) B.) Light: Electromagnetic radiation Two different views of light: 1.) Wave Model ...
... A.) Spectroscopy: A method of analysis based on the interaction, absorption or production of light by matter. (also may include the interaction of electrons, ions or acoustics with matter) B.) Light: Electromagnetic radiation Two different views of light: 1.) Wave Model ...
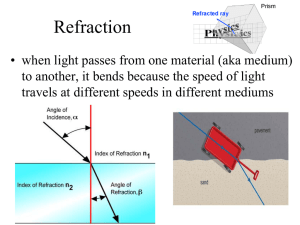
The Index of Refraction and Snell`s Law
... Snell’s Law relates the angle of incidence and the angle of refraction in the two different media. ...
... Snell’s Law relates the angle of incidence and the angle of refraction in the two different media. ...
Fun Physics You Can See
... from different atoms and molecules. • Electrons in an atom can get excited. An excited electron is in a state of high energy. Sometimes these electrons jump down to a state with low energy. The difference in energy is given off as light. ...
... from different atoms and molecules. • Electrons in an atom can get excited. An excited electron is in a state of high energy. Sometimes these electrons jump down to a state with low energy. The difference in energy is given off as light. ...
Ch. 19 - Optics 19.1 MIRRORS Light is made up of rays that travel in
... How much the speed of light rays slows as it enters a new material depends on the material‛s index of refraction - a measure of how much light changes speed as it enters a new medium. It is calculated by finding the ratio of the speed of light in a vacuum to the speed of light in a material. For exa ...
... How much the speed of light rays slows as it enters a new material depends on the material‛s index of refraction - a measure of how much light changes speed as it enters a new medium. It is calculated by finding the ratio of the speed of light in a vacuum to the speed of light in a material. For exa ...
Dispersion of Light by Prisms In the Light and Color unit of The
... The optical density of a material is the result of the tendency of the atoms of a material to maintain the absorbed energy of the light wave in the form of vibrating electrons before reemitting it as a new electromagnetic disturbance. Thus, while a light wave travels through a vacuum at a speed of c ...
... The optical density of a material is the result of the tendency of the atoms of a material to maintain the absorbed energy of the light wave in the form of vibrating electrons before reemitting it as a new electromagnetic disturbance. Thus, while a light wave travels through a vacuum at a speed of c ...
PP The origins of colour
... For example, copper(II) ions have only nine electrons in their 3d sub-level. The horizontal line represents their energy level before binding to a ligand. ...
... For example, copper(II) ions have only nine electrons in their 3d sub-level. The horizontal line represents their energy level before binding to a ligand. ...
Properties of optically
... mirror images of each other and cannot be superimposed — just as your left and right hands are mirror images and cannot be superimposed. The structure for glucose shows four chiral centres, which means a total of 16 different forms are possible. Glucose is just one of those forms. ...
... mirror images of each other and cannot be superimposed — just as your left and right hands are mirror images and cannot be superimposed. The structure for glucose shows four chiral centres, which means a total of 16 different forms are possible. Glucose is just one of those forms. ...
It`s Bent II
... With a transparent object (air, water, clear glass) almost all light passes through. As light passes from one transparent material to another at an angle (from air to water, or air to glass), the light will slow down and appear bent. This is called refraction. A good example of this is placing a pen ...
... With a transparent object (air, water, clear glass) almost all light passes through. As light passes from one transparent material to another at an angle (from air to water, or air to glass), the light will slow down and appear bent. This is called refraction. A good example of this is placing a pen ...
Honors Physics – Refraction Supplemental Problems A ray of light
... 8. Draw the path that the light would take through the prism for the above situation. 9. Find the distance by which the original ray deviates from its original pathafter it has refracted while passing through the block shown below. Consider that the block is in air and has an index of refraction ...
... 8. Draw the path that the light would take through the prism for the above situation. 9. Find the distance by which the original ray deviates from its original pathafter it has refracted while passing through the block shown below. Consider that the block is in air and has an index of refraction ...
93d6fd332e7f2a99ea9a02db430ccf53ef4dfc59
... What are suspensions? homogeneous mixtures with particles that have diameters greater than 1000 nm, 0.000001 meter What are 2 examples of suspensions? Blood and aerosol sprays Go to: http://www.sciencehq.com/chemistry/properties-of-colloidal-solution.html What is Brownian Motion/Movement and what ca ...
... What are suspensions? homogeneous mixtures with particles that have diameters greater than 1000 nm, 0.000001 meter What are 2 examples of suspensions? Blood and aerosol sprays Go to: http://www.sciencehq.com/chemistry/properties-of-colloidal-solution.html What is Brownian Motion/Movement and what ca ...
Synthetic Polymers - McQuarrie General Chemistry
... molecules incorporated into a polymer molecule is not important for typically large values of n. Polymer syntheses generally produce polymer molecules with a range of n values. The polymer properties are described in terms of the average value of n. It makes little difference whether a polyethylene ...
... molecules incorporated into a polymer molecule is not important for typically large values of n. Polymer syntheses generally produce polymer molecules with a range of n values. The polymer properties are described in terms of the average value of n. It makes little difference whether a polyethylene ...
The benefiTs and dangers of blue lighT for your
... lenses use an exclusive technology to selectively filter light. UV rays and harmful blue-violet light are virtually eliminated. Essential BlueTurquoise light passes through. The lens is clear and can be worn for visual correction. Ask your eye care professional for more details. ...
... lenses use an exclusive technology to selectively filter light. UV rays and harmful blue-violet light are virtually eliminated. Essential BlueTurquoise light passes through. The lens is clear and can be worn for visual correction. Ask your eye care professional for more details. ...
Light - brown09
... Light is very important in everybody’s life. It is one of the many ways that energy can travel. Moving through space, water, or other materials in waves, it lets you see everything around you that is lit up. Millions of light photons bounce off of the objects that the light reaches and they go in ev ...
... Light is very important in everybody’s life. It is one of the many ways that energy can travel. Moving through space, water, or other materials in waves, it lets you see everything around you that is lit up. Millions of light photons bounce off of the objects that the light reaches and they go in ev ...
Apparent Depth
... around 300,000 kilometres per second. At this speed it can go around the world 8 times in one second. ...
... around 300,000 kilometres per second. At this speed it can go around the world 8 times in one second. ...
Photopolymer
A photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing,.A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize in the presence of light either through internal or external initiation. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo curing is the formation of a thermoset network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene and oligomeric acrylates.Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet light is more energetic; however, the development of dye-based photoinitiator systems have allowed for the use of visible light, having potential advantages of processes that are more simple and safe to handle. UV curing in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization include high rates of polymerization and environmental benefits from elimination of volatile organic solvents.There are two general routes for photoinitiation: free radical and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.