UV-Vis Spectroscopy
... 1st choice in most laboratories concerned with the identification and measurement of organic and inorganic compounds - in nucleic acids, proteins, foodstuffs, pharmaceuticals and fertilizers, in mineral oils and in paint. In every branch of molecular biology, medicine and the life sciences, th ...
... 1st choice in most laboratories concerned with the identification and measurement of organic and inorganic compounds - in nucleic acids, proteins, foodstuffs, pharmaceuticals and fertilizers, in mineral oils and in paint. In every branch of molecular biology, medicine and the life sciences, th ...
Physics David Sang - Assets - Cambridge University Press
... Most of us look in a mirror at least once a day, to check on our appearance (Figure 13.7). It is important to us to know that we are presenting ourselves to the rest of the world in the way we want. Archaeologists have found bronze mirrors over 2000 years old, so the desire to see ourselves clearly ...
... Most of us look in a mirror at least once a day, to check on our appearance (Figure 13.7). It is important to us to know that we are presenting ourselves to the rest of the world in the way we want. Archaeologists have found bronze mirrors over 2000 years old, so the desire to see ourselves clearly ...
Chapter 2 Using Light - Red Hill Lutheran School
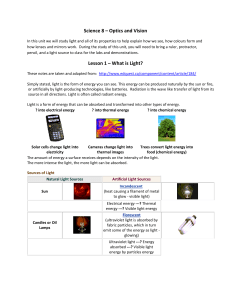
... What causes waves? What are the basic properties of waves? What does an electromagnetic wave consist of? What are the waves of the electromagnetic spectrum? ...
... What causes waves? What are the basic properties of waves? What does an electromagnetic wave consist of? What are the waves of the electromagnetic spectrum? ...
Q1. (a) The diagram below shows the path followed by a light ray
... (a) Light has a dual wave-particle nature. State and outline a piece of evidence for the wave nature of light and a piece of evidence for its particle nature. For each piece of evidence, outline a characteristic feature that has been observed or measured and give a short explanation of its relevance ...
... (a) Light has a dual wave-particle nature. State and outline a piece of evidence for the wave nature of light and a piece of evidence for its particle nature. For each piece of evidence, outline a characteristic feature that has been observed or measured and give a short explanation of its relevance ...
The Properties of Light and Color – What we see
... To interpret ray diagrams there are a few key things you must take into consideration. First you must look to see that light is traveling in all directions. Second you must see if the light rays are traveling in a straight direction. And third you must determine where light is reflected, and where l ...
... To interpret ray diagrams there are a few key things you must take into consideration. First you must look to see that light is traveling in all directions. Second you must see if the light rays are traveling in a straight direction. And third you must determine where light is reflected, and where l ...
9.1-10.5 Organic Chemistry
... Number the carbon atoms, starting from the end closest to the branch(es) so that the numbers are the lowest possible Identify any branches and their location number on the parent chain (us the suffix –yl for branches) Write the complete IUPAC name, following the format: (number of ...
... Number the carbon atoms, starting from the end closest to the branch(es) so that the numbers are the lowest possible Identify any branches and their location number on the parent chain (us the suffix –yl for branches) Write the complete IUPAC name, following the format: (number of ...
Lab #10
... We will explore the ideas of refraction in class, but your main experience with it will be in this lab. In this lab you will explore and verify a quantitative relationship that describes the path of light in different media. You’ll also learn how the ideas of refraction connect to fiber optics and b ...
... We will explore the ideas of refraction in class, but your main experience with it will be in this lab. In this lab you will explore and verify a quantitative relationship that describes the path of light in different media. You’ll also learn how the ideas of refraction connect to fiber optics and b ...
Properties of Light I
... the colour associated with that wavelength ERSC 2P22 - Brock University Greg Finn ...
... the colour associated with that wavelength ERSC 2P22 - Brock University Greg Finn ...
Optics - hrsbstaff.ednet.ns.ca
... Chapt22, Conceptual-1: Under certain circumstances, sound can be heard from extremely far away. This frequently happens over a body of water, where the air near the water surface is cooler than the air at higher altitudes. Explain how the refraction of sound waves could increase the distance over wh ...
... Chapt22, Conceptual-1: Under certain circumstances, sound can be heard from extremely far away. This frequently happens over a body of water, where the air near the water surface is cooler than the air at higher altitudes. Explain how the refraction of sound waves could increase the distance over wh ...
Chemistry Mid-Term Review Guide
... Separating Mixtures (cont.) • Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids when one sublimates and the other does not. • Chromatography is a technique that separates the components of a mixture on the basis of tendency of each to ...
... Separating Mixtures (cont.) • Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids when one sublimates and the other does not. • Chromatography is a technique that separates the components of a mixture on the basis of tendency of each to ...
Chemistry: Matter and Change
... Separating Mixtures (cont.) • Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids when one sublimates and the other does not. • Chromatography is a technique that separates the components of a mixture on the basis of tendency of each to ...
... Separating Mixtures (cont.) • Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids when one sublimates and the other does not. • Chromatography is a technique that separates the components of a mixture on the basis of tendency of each to ...
Index of Refraction
... In addition to the dependency of the index of refraction on the frequency of radiation, there is an additional complication: the index of refraction can also depend on the direction the light is moving through the material. This phenomenon occurs in crystals such as Iceland spar (calcite, CaCO3). Cr ...
... In addition to the dependency of the index of refraction on the frequency of radiation, there is an additional complication: the index of refraction can also depend on the direction the light is moving through the material. This phenomenon occurs in crystals such as Iceland spar (calcite, CaCO3). Cr ...
Nick Shumaker - HawksPhysicalScienceBlue2
... refract the medium. What are lasers used for? Lasers can be used for many things like aming weapons to be more accurate, or to break down materials. Where does light occur? Light occurs in almost all places except for black holes. What are the different types of lasers? A few types of ...
... refract the medium. What are lasers used for? Lasers can be used for many things like aming weapons to be more accurate, or to break down materials. Where does light occur? Light occurs in almost all places except for black holes. What are the different types of lasers? A few types of ...
EMR notes
... and magnetic fields that are varying with time and travelling away from their source at the speed of light Faraday believed in unity in nature and that there was a relationship between light, electricity and magnetism. He however, lacked the mathematics to prove this. James Clerk Maxwell, in 1860’s, ...
... and magnetic fields that are varying with time and travelling away from their source at the speed of light Faraday believed in unity in nature and that there was a relationship between light, electricity and magnetism. He however, lacked the mathematics to prove this. James Clerk Maxwell, in 1860’s, ...
Photopolymer
A photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing,.A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize in the presence of light either through internal or external initiation. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo curing is the formation of a thermoset network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene and oligomeric acrylates.Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet light is more energetic; however, the development of dye-based photoinitiator systems have allowed for the use of visible light, having potential advantages of processes that are more simple and safe to handle. UV curing in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization include high rates of polymerization and environmental benefits from elimination of volatile organic solvents.There are two general routes for photoinitiation: free radical and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.