Chapter 13 ppt.
... facing mirrors at opposite ends of the laser. • One of the mirrors is coated only partially with reflective material, so it reflects most light but allows some to get through. • Some emitted light waves travel back and forth between the mirrors many times, stimulating other atoms to emit identical l ...
... facing mirrors at opposite ends of the laser. • One of the mirrors is coated only partially with reflective material, so it reflects most light but allows some to get through. • Some emitted light waves travel back and forth between the mirrors many times, stimulating other atoms to emit identical l ...
File
... Conservation of Matter). This states that matter is neither created nor destroyed. What this means to us is that whatever mass you start out with is the same amount of mass you end up with. For example, in the above equation if you start with 5 g of Fe and end up producing 6.2 g of Fe2O3 you will ha ...
... Conservation of Matter). This states that matter is neither created nor destroyed. What this means to us is that whatever mass you start out with is the same amount of mass you end up with. For example, in the above equation if you start with 5 g of Fe and end up producing 6.2 g of Fe2O3 you will ha ...
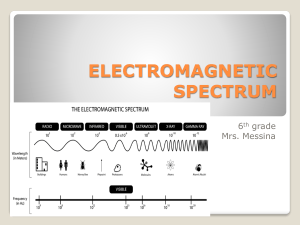
electromagnetic spectrum - White Plains Public Schools
... spectrum, a fact that can be easily proven through the use of a prism. As light passes through a prism, it is bent, or refracted, by the angles and plane faces of the prism and each wavelength of light is refracted by a slightly different amount. Violet has the highest frequency and is refracted the ...
... spectrum, a fact that can be easily proven through the use of a prism. As light passes through a prism, it is bent, or refracted, by the angles and plane faces of the prism and each wavelength of light is refracted by a slightly different amount. Violet has the highest frequency and is refracted the ...
anna-chrobok-silesian-university-of-technology
... - Diels-Alder reaction, - oxidation of alcohols and ketones. IONIC LIQUIDS as homogeneous and heterogeneous catalysts Recycling of ionic liquids prevents them from: - ending up in the aquatic environment, - release into the atmosphere (low volatility). ...
... - Diels-Alder reaction, - oxidation of alcohols and ketones. IONIC LIQUIDS as homogeneous and heterogeneous catalysts Recycling of ionic liquids prevents them from: - ending up in the aquatic environment, - release into the atmosphere (low volatility). ...
Mixtures
... Common Techniques for Separating Mixtures Distillation- a process that separates a mixture based ...
... Common Techniques for Separating Mixtures Distillation- a process that separates a mixture based ...
H 2 O
... oxidize or reduce • Although both oxidizing and reducing radicals are produced in solvents by ionizing radiation, one or the other can usually be selectively scavenged. eaq + N2O N2 + O ...
... oxidize or reduce • Although both oxidizing and reducing radicals are produced in solvents by ionizing radiation, one or the other can usually be selectively scavenged. eaq + N2O N2 + O ...
19-2 What is light?
... 6. Would it be possible for you to see the star Betelgeuse, even if that star had died hundreds of years ago? Explain your answer. ___________________________________________________________________ ___________________________________________________________________________________________ _________ ...
... 6. Would it be possible for you to see the star Betelgeuse, even if that star had died hundreds of years ago? Explain your answer. ___________________________________________________________________ ___________________________________________________________________________________________ _________ ...
Investigating Light - Discover Primary Science
... lenses – one short focal length (say 5cm) and one long focal length (say 20 cm), sellotape. ...
... lenses – one short focal length (say 5cm) and one long focal length (say 20 cm), sellotape. ...
What are Physical Properties and Changes? - Mamanakis
... Another sign of a chemical change is the release or as it changes form a gain of energy by an object. Many substances runny mix into a cake. absorb energy to undergo a chemical change. Energy is absorbed during chemical changes involved in cooking, like baking a cake. Energy can also be released dur ...
... Another sign of a chemical change is the release or as it changes form a gain of energy by an object. Many substances runny mix into a cake. absorb energy to undergo a chemical change. Energy is absorbed during chemical changes involved in cooking, like baking a cake. Energy can also be released dur ...
Basic Optics
... In 1960, the first Ruby laser was built [1], opening the door for many new optical inventions and research topics. Mechanical engineers in particular are currently working on optical methods for measuring fluid and gas properties, measuring mechanical stresses, and manufacturing techniques. In order ...
... In 1960, the first Ruby laser was built [1], opening the door for many new optical inventions and research topics. Mechanical engineers in particular are currently working on optical methods for measuring fluid and gas properties, measuring mechanical stresses, and manufacturing techniques. In order ...
19/Light and Sound in the Sea
... are sometimes located on the undersides of fish, which may serve some camouflage purpose. Many species of viperfish and anglerfish that live in deeper waters have bioluminescent lures that glow in the dark, an adaptation for attracting prey. Organisms may also use bioluminescence to see in the dark, ...
... are sometimes located on the undersides of fish, which may serve some camouflage purpose. Many species of viperfish and anglerfish that live in deeper waters have bioluminescent lures that glow in the dark, an adaptation for attracting prey. Organisms may also use bioluminescence to see in the dark, ...
Microscopes I - Sewanhaka Central High School District
... The first useful microscope was developed in the Netherlands in the early 1600s ...
... The first useful microscope was developed in the Netherlands in the early 1600s ...
The Effect of Light Proximity On the Rate of Photosynthesis
... leaf disks were placed into cups filled with a sodium bicarbonate mixture. Three of these cups were placed at different distances from a clamp light containing a 42Watt fluorescent light bulb. It was found that the disks in the cup closest to the light seemed to have risen the fastest, while the cup ...
... leaf disks were placed into cups filled with a sodium bicarbonate mixture. Three of these cups were placed at different distances from a clamp light containing a 42Watt fluorescent light bulb. It was found that the disks in the cup closest to the light seemed to have risen the fastest, while the cup ...
Journal of Molecular Catalysis A: Chemical Enhancing
... when treated with sodium bromide afforded corresponding bromide that exhibited doublet at ı 6.61 (J = 12.4 Hz) indicating the presence of double bond [33] and upfield shift in the signal of saturated methylene protons containing alcoholic group. Monohalogenation of diols [34] is often a stubborn prob ...
... when treated with sodium bromide afforded corresponding bromide that exhibited doublet at ı 6.61 (J = 12.4 Hz) indicating the presence of double bond [33] and upfield shift in the signal of saturated methylene protons containing alcoholic group. Monohalogenation of diols [34] is often a stubborn prob ...
Noninvasive monitoring with strongly absorbed light
... the “background visible” signal in Fig. 8. This component is also evident in the “probe visible” signal. Testing during development of the instrument described here, demonstrated that periodic components, due to background lighting, were evident even when thick black cloth was placed over the probe. ...
... the “background visible” signal in Fig. 8. This component is also evident in the “probe visible” signal. Testing during development of the instrument described here, demonstrated that periodic components, due to background lighting, were evident even when thick black cloth was placed over the probe. ...
Sub Unit Plan 1 Chem Periodic Table
... II.3 Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases. (3.1v) II.4 Elements can be differentiated by their physical properties. Physical properties of substances, such as density, conductivity, ...
... II.3 Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases. (3.1v) II.4 Elements can be differentiated by their physical properties. Physical properties of substances, such as density, conductivity, ...
Ch1small - Rutgers University
... Extensive properties: depend on amount of material present (mass, volume). Physical and Chemical Changes Physical changes do not effect the composition of the material. Most common is change of state such as evaporation (vaporization: liquid to gas fusion: liquid to solid sublimation: solid to gas) ...
... Extensive properties: depend on amount of material present (mass, volume). Physical and Chemical Changes Physical changes do not effect the composition of the material. Most common is change of state such as evaporation (vaporization: liquid to gas fusion: liquid to solid sublimation: solid to gas) ...
Lecture Notes 1 - Rutgers University
... Extensive properties: depend on amount of material present (mass, volume). Physical and Chemical Changes Physical changes do not effect the composition of the material. Most common is change of state such as evaporation (vaporization: liquid to gas fusion: liquid to solid sublimation: solid to gas) ...
... Extensive properties: depend on amount of material present (mass, volume). Physical and Chemical Changes Physical changes do not effect the composition of the material. Most common is change of state such as evaporation (vaporization: liquid to gas fusion: liquid to solid sublimation: solid to gas) ...
Topic: Refraction of Light
... T: If the light ray did not approach to a mirror but to a surface of water, please guess what will happen? S: Some of the light ray is reflected back into air, the other is traveling into water. T: The light ray in the water will be near or away from the normal or the direction will not change? Stud ...
... T: If the light ray did not approach to a mirror but to a surface of water, please guess what will happen? S: Some of the light ray is reflected back into air, the other is traveling into water. T: The light ray in the water will be near or away from the normal or the direction will not change? Stud ...
ert207 analytical chemistry
... • The stationary phase comes in the form of a packed syringe-shaped cartridge • Solid phase extraction cartridges and disks are available with a variety of stationary phases, each of which can separate analytes according to different chemical properties. • Most stationary phases are based on silica ...
... • The stationary phase comes in the form of a packed syringe-shaped cartridge • Solid phase extraction cartridges and disks are available with a variety of stationary phases, each of which can separate analytes according to different chemical properties. • Most stationary phases are based on silica ...
Photopolymer
A photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing,.A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize in the presence of light either through internal or external initiation. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo curing is the formation of a thermoset network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene and oligomeric acrylates.Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet light is more energetic; however, the development of dye-based photoinitiator systems have allowed for the use of visible light, having potential advantages of processes that are more simple and safe to handle. UV curing in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization include high rates of polymerization and environmental benefits from elimination of volatile organic solvents.There are two general routes for photoinitiation: free radical and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.