here - UFL MAE - University of Florida
... Required Text: Fundamentals of Heat and Mass Transfer, F.P. Incropera, D.P. DeWitt, T.L. Bergman, and A.S. Lavine 6th Edition (John Wiley & Sons, 2007) Course Website: Sakai Prereq: EML 3100, EGM 4313 Meeting Time & Place: M-W-F, 5th Period, Room 0270 WEIL Course Objectives and Outcomes: This course ...
... Required Text: Fundamentals of Heat and Mass Transfer, F.P. Incropera, D.P. DeWitt, T.L. Bergman, and A.S. Lavine 6th Edition (John Wiley & Sons, 2007) Course Website: Sakai Prereq: EML 3100, EGM 4313 Meeting Time & Place: M-W-F, 5th Period, Room 0270 WEIL Course Objectives and Outcomes: This course ...
Power point about heat transfer
... • The heat in the air is moving from the air into the popsicle. This is because heat always moves in the direction from higher temperatures to a lower temperatures. ...
... • The heat in the air is moving from the air into the popsicle. This is because heat always moves in the direction from higher temperatures to a lower temperatures. ...
PPT Slide Show
... Types of Heat Transfer • Heat transfer explains the transfer of thermal energy, between physical systems depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation. ...
... Types of Heat Transfer • Heat transfer explains the transfer of thermal energy, between physical systems depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation. ...
Ideal Gas Law / Heat Transfer
... But what if the two objects were different sizes? Lake Michigan vs. cup of water Which one do you need to add more heat to in order to raise the temperature? ...
... But what if the two objects were different sizes? Lake Michigan vs. cup of water Which one do you need to add more heat to in order to raise the temperature? ...
What is the DSC used for
... sensor or between the sensor and sample pan. The cell sensor consists of a constantan body with separate raised platforms or vessel to hold the sample and reference (Fig. 1). The platforms are connected to the heating block (base) by thin-walled tubes that create thermal resistances between the plat ...
... sensor or between the sensor and sample pan. The cell sensor consists of a constantan body with separate raised platforms or vessel to hold the sample and reference (Fig. 1). The platforms are connected to the heating block (base) by thin-walled tubes that create thermal resistances between the plat ...
What is the DSC used for
... sensor or between the sensor and sample pan. The cell sensor consists of a constantan body with separate raised platforms or vessel to hold the sample and reference (Fig. 1). The platforms are connected to the heating block (base) by thin-walled tubes that create thermal resistances between the plat ...
... sensor or between the sensor and sample pan. The cell sensor consists of a constantan body with separate raised platforms or vessel to hold the sample and reference (Fig. 1). The platforms are connected to the heating block (base) by thin-walled tubes that create thermal resistances between the plat ...
Kotze_J - Energy Postgraduate Conference 2013
... steam power block to a ultra supercritical steam power block: – 26.3% savings in thermal input from the heliostats. – 26.3% less heliostats – And a 5.8% reduction in LCOE, and a 9.8% reduction in plant cost based on heliostats alone. ...
... steam power block to a ultra supercritical steam power block: – 26.3% savings in thermal input from the heliostats. – 26.3% less heliostats – And a 5.8% reduction in LCOE, and a 9.8% reduction in plant cost based on heliostats alone. ...
Step 4: Cut along the 2 fold lines to make 3 flaps
... Draw these lines where you made the cuts to separate these sections and write these 3 words on the tabs. (Do not write the words Cold are just the instructions.) Hotin red. These ...
... Draw these lines where you made the cuts to separate these sections and write these 3 words on the tabs. (Do not write the words Cold are just the instructions.) Hotin red. These ...
Thermal Mass
... •SUNREL (free on request) A general-purpose thermal analysis program for residential buildings. The solution approach is a thermal network using a combination of forward finite differencing, Jacobian iteration, and constrained optimization. It was written to aid in the design of small energy efficie ...
... •SUNREL (free on request) A general-purpose thermal analysis program for residential buildings. The solution approach is a thermal network using a combination of forward finite differencing, Jacobian iteration, and constrained optimization. It was written to aid in the design of small energy efficie ...
Part II. Convection Currents and the Mantle
... 3) ____Can heat transfer by radiation go through a wall? C. Go to www.universetoday.com/26717/earths-mantle/ to learn about what influence convection currents have on Earth. Read and answer the following questions. 1. Describe how convection currents occur in Earth’s mantle. ________________________ ...
... 3) ____Can heat transfer by radiation go through a wall? C. Go to www.universetoday.com/26717/earths-mantle/ to learn about what influence convection currents have on Earth. Read and answer the following questions. 1. Describe how convection currents occur in Earth’s mantle. ________________________ ...
Golden Valley HS • AP Chemistry
... 1 calorie = 4.184 J This is also the specific heat of water (4.184 J/goC). Specific heat of other substances is defined as the energy needed to raise 1 gram of the substance 1oC. Substances with larger specific heat values take more energy to change the temperature. They also hold their heat longer ...
... 1 calorie = 4.184 J This is also the specific heat of water (4.184 J/goC). Specific heat of other substances is defined as the energy needed to raise 1 gram of the substance 1oC. Substances with larger specific heat values take more energy to change the temperature. They also hold their heat longer ...
Proudly Presents: Dr. Mark Hepokoski
... Dr. Hepokoski is the Director of Advanced Research at ThermoAnalytics. As a principal investigator of human thermal physiology and comfort projects, he has developed and validated a complex model of human thermo-physiology, which is widely used in industry and academia for predicting human thermal s ...
... Dr. Hepokoski is the Director of Advanced Research at ThermoAnalytics. As a principal investigator of human thermal physiology and comfort projects, he has developed and validated a complex model of human thermo-physiology, which is widely used in industry and academia for predicting human thermal s ...
Unit 7 Study Guide Answer Key
... 17. Several days after a snowfall, the roofs of some home on a street have almost no snow on them, while the roofs of other homes are still snow-covered. Give one reason, related to home insulation, that might cause this difference. The homes with snow have insulation in their roof while those witho ...
... 17. Several days after a snowfall, the roofs of some home on a street have almost no snow on them, while the roofs of other homes are still snow-covered. Give one reason, related to home insulation, that might cause this difference. The homes with snow have insulation in their roof while those witho ...
Transferts couplés de masse et de chaleur dans une mousse solide
... knowledge of all mechanisms which govern heat and mass transfers during various processes: baking, chilling and eventually freezing and thawing. This paper presents a synthesis of works achieved to fill this gap. The objective is to develop a coupled heat and mass transfers model in the case of soli ...
... knowledge of all mechanisms which govern heat and mass transfers during various processes: baking, chilling and eventually freezing and thawing. This paper presents a synthesis of works achieved to fill this gap. The objective is to develop a coupled heat and mass transfers model in the case of soli ...
Schaums Heat
... 13. An electric heater that produces 900 W of power is used to vaporize water. How much water at 100 0C can be changed to steam at 1000C in 3 minutes by the heater? 14. A 3 gram bullet (c = 128 J/kgC) moving at 180 m/s enters a bag of sand and stops. By what amount does the temperature of the bullet ...
... 13. An electric heater that produces 900 W of power is used to vaporize water. How much water at 100 0C can be changed to steam at 1000C in 3 minutes by the heater? 14. A 3 gram bullet (c = 128 J/kgC) moving at 180 m/s enters a bag of sand and stops. By what amount does the temperature of the bullet ...
PASSIVE DESIGN Introduction Theory
... Regarding insulation, the heat loss through the construction (walls, floor, basement, ceiling or the roof) is represented by the thermal heat loss coefficient or U-value, which represents how much heat in Watts is lost per m2 at a standard temperature difference. Insulation materials are normally us ...
... Regarding insulation, the heat loss through the construction (walls, floor, basement, ceiling or the roof) is represented by the thermal heat loss coefficient or U-value, which represents how much heat in Watts is lost per m2 at a standard temperature difference. Insulation materials are normally us ...
Chapters 12-15 Thermodynamics
... • Block of ice and the balloon are each in mechanical equilibrium: F = 0 • Now put them in contact • Both systems now undergo changes • The volume of ice decreases (it melts) and the pressure and volume of the balloon decrease • They come to a final state of thermal equilibrium which must be chara ...
... • Block of ice and the balloon are each in mechanical equilibrium: F = 0 • Now put them in contact • Both systems now undergo changes • The volume of ice decreases (it melts) and the pressure and volume of the balloon decrease • They come to a final state of thermal equilibrium which must be chara ...
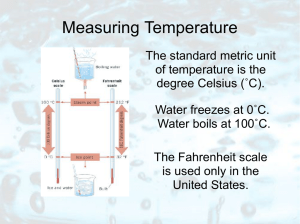
HEAT ENERGY
... The temperature of an object tells us how HOT it is Measured in degrees Celsius - °C It is NOT the same as heat energy although the two quantities are related. e.g. a beaker of water at 60 °C is hotter than a bath of water at 40 °C BUT the bath contains more joules of heat energy ...
... The temperature of an object tells us how HOT it is Measured in degrees Celsius - °C It is NOT the same as heat energy although the two quantities are related. e.g. a beaker of water at 60 °C is hotter than a bath of water at 40 °C BUT the bath contains more joules of heat energy ...