Electronics Cooling MEP 635
... • Introduction to electronics cooling and thermal packaging • Introduction to basic modes of heat transfer • Conduction heat transfer and extended surfaces in electronic devices • Transient conduction • Natural convection heat transfer (i.e. PCB cooling) • Forced convection heat transfer (Internal a ...
... • Introduction to electronics cooling and thermal packaging • Introduction to basic modes of heat transfer • Conduction heat transfer and extended surfaces in electronic devices • Transient conduction • Natural convection heat transfer (i.e. PCB cooling) • Forced convection heat transfer (Internal a ...
Temperature in Thermal Systems
... • Thermometer – device used to measure temperature; uses the expansion and contraction of a liquid, usu. colored alcohol or mercury (Hg). ...
... • Thermometer – device used to measure temperature; uses the expansion and contraction of a liquid, usu. colored alcohol or mercury (Hg). ...
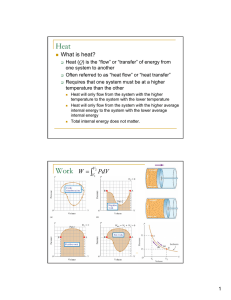
Heat Work
... Heat added +, heat lost -, work done by system +, work done on system – Internal Energy U is a state property Work W and heat Q are not But work and heat are involved in thermodynamic processes that change the state of the system ...
... Heat added +, heat lost -, work done by system +, work done on system – Internal Energy U is a state property Work W and heat Q are not But work and heat are involved in thermodynamic processes that change the state of the system ...
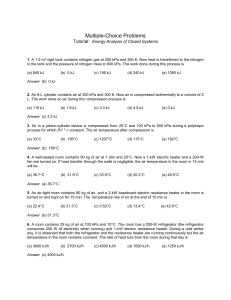
Multiple Choice Questions_1
... 2. An 8-L cylinder contains air at 300 kPa and 300 K. Now air is compressed isothermally to a volume of 2 L. The work done on air during this compression process is (a) 119 kJ ...
... 2. An 8-L cylinder contains air at 300 kPa and 300 K. Now air is compressed isothermally to a volume of 2 L. The work done on air during this compression process is (a) 119 kJ ...
3. The elements of the buildings` envelope
... As low insulated slabs over basements or ground can account for 10% to 15% of the heat losses of a house, it is necessary to foresee a thermal insulation on the first slab under heated space. If the basement is heated, thermal insulation should be installed on the slab over ground. If the basement i ...
... As low insulated slabs over basements or ground can account for 10% to 15% of the heat losses of a house, it is necessary to foresee a thermal insulation on the first slab under heated space. If the basement is heated, thermal insulation should be installed on the slab over ground. If the basement i ...
2003 ME Graduate Student Conference
... parameter. The dimensionless Knudsen number determines which convective heat transfer coefficient must be used in the simulation. The ambient pressure will change the Knudsen number and as a result, the flow regime. Several researchers have studied gas pressure effects on heat transfer from the mico ...
... parameter. The dimensionless Knudsen number determines which convective heat transfer coefficient must be used in the simulation. The ambient pressure will change the Knudsen number and as a result, the flow regime. Several researchers have studied gas pressure effects on heat transfer from the mico ...
Summer Heat Protection
... is apparent in the research of Professor Hauser, one of the founding fathers of German energy efficiency. Using the example of a one bedroom house we are able to reduce the “uncomfortable hours” by swapping the mineral wool for Wood Fibre insulation by almost 50 %. To put it simply, one sweats a lot ...
... is apparent in the research of Professor Hauser, one of the founding fathers of German energy efficiency. Using the example of a one bedroom house we are able to reduce the “uncomfortable hours” by swapping the mineral wool for Wood Fibre insulation by almost 50 %. To put it simply, one sweats a lot ...
Thermodynamics–Honors
... ΔΕ= positive (+), energy gained by system ΔΕ= negative (-), energy lost by system Total energy = sum of the energy of each part in a chemical reaction ...
... ΔΕ= positive (+), energy gained by system ΔΕ= negative (-), energy lost by system Total energy = sum of the energy of each part in a chemical reaction ...
saving with heat pumps
... your zone—zones 1 and 2 (map below) are generally warm enough to allow heat pumps to operate efficiently. Generally, the yearly average ambient temperature should be 19 degrees Celsius or higher how many STCs—the number of STCs (or Small-scale Technology Certificates) for each system also depends on ...
... your zone—zones 1 and 2 (map below) are generally warm enough to allow heat pumps to operate efficiently. Generally, the yearly average ambient temperature should be 19 degrees Celsius or higher how many STCs—the number of STCs (or Small-scale Technology Certificates) for each system also depends on ...
Specific Heat Equation Practice Worksheet
... c. Determine the heat capacity of a substance using mass, specific heat, and temperature. You have probably noticed that a metal spoon heats up quickly when placed in a cup of soap while a plastic spoon heats more slowly. The difference between the final temperatures of the two spoons depends on whe ...
... c. Determine the heat capacity of a substance using mass, specific heat, and temperature. You have probably noticed that a metal spoon heats up quickly when placed in a cup of soap while a plastic spoon heats more slowly. The difference between the final temperatures of the two spoons depends on whe ...
Specific Heat WS #2 - My Chemistry Class
... What would be the final temperature of a 73.174g sample of cobalt with an initial temperature of 102.0 °C, after it loses 6800 J? (Note the specific heat of cobalt is 0.4210 J/g°C) ...
... What would be the final temperature of a 73.174g sample of cobalt with an initial temperature of 102.0 °C, after it loses 6800 J? (Note the specific heat of cobalt is 0.4210 J/g°C) ...
Flat Plate Boundary Layer
... aluminum tubes. The coolant flows from the inlet to the outlet through many tubes mounted in a parallel arrangement. The fins conduct the heat from the tubes and transfer it to the air flowing through the radiator. The tubes sometimes have a type of fin inserted into them called a turbulator, which ...
... aluminum tubes. The coolant flows from the inlet to the outlet through many tubes mounted in a parallel arrangement. The fins conduct the heat from the tubes and transfer it to the air flowing through the radiator. The tubes sometimes have a type of fin inserted into them called a turbulator, which ...
WORKSHOP 2: Dimensional Analysis and
... 7. A rubber stopper weighing 65.4 g is immersed into a graduated cylinder filled with 30.0 mL of liquid. The liquid level then rises to 48.8 mL. Calculate the density of the stopper. 3.48 g/mL 8. If the density of a liquid is known to be 0.785 g/mL, calculate the mass of the liquid if its volume is ...
... 7. A rubber stopper weighing 65.4 g is immersed into a graduated cylinder filled with 30.0 mL of liquid. The liquid level then rises to 48.8 mL. Calculate the density of the stopper. 3.48 g/mL 8. If the density of a liquid is known to be 0.785 g/mL, calculate the mass of the liquid if its volume is ...
Pioneer Science Worksheet
... 1. It is often thought that heat and temperature is the same thing. Although related to each other, heat and temperature have different concepts. The following are true about ...
... 1. It is often thought that heat and temperature is the same thing. Although related to each other, heat and temperature have different concepts. The following are true about ...
Thermodynamics
... When a system gains heat, the internal energy of the system increases. Q is positive when a system gains heat and negative when a system loses heat. Internal energy of a system can decrease if the system does work on its surroundings. Work is positive when it is done by the system and negative when ...
... When a system gains heat, the internal energy of the system increases. Q is positive when a system gains heat and negative when a system loses heat. Internal energy of a system can decrease if the system does work on its surroundings. Work is positive when it is done by the system and negative when ...
state of matter - Mayfield City Schools
... -What we’ve learned thus far about heat and thermal energy is summed up in the laws of thermodynamics. The word thermodynamics stems from Greek for “movement of heat.” -When thermal energy transfers as heat, it does so without net loss or gain. The energy lost from one place is gained by the other. ...
... -What we’ve learned thus far about heat and thermal energy is summed up in the laws of thermodynamics. The word thermodynamics stems from Greek for “movement of heat.” -When thermal energy transfers as heat, it does so without net loss or gain. The energy lost from one place is gained by the other. ...
Heat Transfer
... An engineer wishes to determine the specific heat of a new metal alloy. A 0.150-kg sample of the alloy is heated to 540°C. It is then quickly placed in 0.400 kg of water at 10.0°C, which is contained in a 0.200-kg aluminum calorimeter cup. (We do not need to know the mass of the insulating jacket si ...
... An engineer wishes to determine the specific heat of a new metal alloy. A 0.150-kg sample of the alloy is heated to 540°C. It is then quickly placed in 0.400 kg of water at 10.0°C, which is contained in a 0.200-kg aluminum calorimeter cup. (We do not need to know the mass of the insulating jacket si ...
ABE 484
... 1. Internalize the meaning of the terminology and physical principles associated with heat and mass transfer in agricultural and biological systems. 2. Learn basic transport mechanisms – conduction, convection, radiation, and mass transfer. 3. Learn standard solution techniques with real-world probl ...
... 1. Internalize the meaning of the terminology and physical principles associated with heat and mass transfer in agricultural and biological systems. 2. Learn basic transport mechanisms – conduction, convection, radiation, and mass transfer. 3. Learn standard solution techniques with real-world probl ...
Vocabulary - cloudfront.net
... 4. A 50 gram piece of aluminum is heated to 100C and then dropped into cool water where the aluminum’s temperature drops to 30C. How many calories does the aluminum loose to the water? (Specific heat capacity Al = 0.215 cal/gC). ...
... 4. A 50 gram piece of aluminum is heated to 100C and then dropped into cool water where the aluminum’s temperature drops to 30C. How many calories does the aluminum loose to the water? (Specific heat capacity Al = 0.215 cal/gC). ...
Temperature Conversions
... 4. A 400g glass coffee cup is at room temperature, 20.0ºC. It is then plunged into hot dishwater, 80.0ºC. If the temperature of the cup reaches that of the dishwater, how much heat does the cup absorb? Assume the mass of the dishwater is large enough so its temperature doesn’t change appreciably. ...
... 4. A 400g glass coffee cup is at room temperature, 20.0ºC. It is then plunged into hot dishwater, 80.0ºC. If the temperature of the cup reaches that of the dishwater, how much heat does the cup absorb? Assume the mass of the dishwater is large enough so its temperature doesn’t change appreciably. ...
Examination Heat Transfer
... Calculate the view factors F12, F13, F23,i en F23,o, with F23,i the view factor from the inner side of cylinder 2 to the surrounding room 3 and F23,o from the outer side of cylinder 2 to the surrounding room 3. Make use of the figures on the next page. (F12=0.8, F13=0,15, F23,i = 0,23 en F23,o = 0.9 ...
... Calculate the view factors F12, F13, F23,i en F23,o, with F23,i the view factor from the inner side of cylinder 2 to the surrounding room 3 and F23,o from the outer side of cylinder 2 to the surrounding room 3. Make use of the figures on the next page. (F12=0.8, F13=0,15, F23,i = 0,23 en F23,o = 0.9 ...