WS- Specific heat
... 3. The specific heat of iron is 0.4494 J/g x oC. How much heat is transferred when a 24.7 kg iron ingot is cooled from 880 oC to 13 oC? 4. Determine the specific heat of a certain metal if a 450 gram sample of it loses 34 500 Joules of heat as its temperature drops by 97 oC. 5. 4786 Joules of heat a ...
... 3. The specific heat of iron is 0.4494 J/g x oC. How much heat is transferred when a 24.7 kg iron ingot is cooled from 880 oC to 13 oC? 4. Determine the specific heat of a certain metal if a 450 gram sample of it loses 34 500 Joules of heat as its temperature drops by 97 oC. 5. 4786 Joules of heat a ...
Sample Exam 3
... 9. The term “absolute zero” refers to a) the temperature at which water freezes. b) the temperature at which carbon dioxide freezes. c) the zero point in the Fahrenheit scale. d) the temperature at which all particle motion stops. e) the coldest temperature ever achieved on Earth. ...
... 9. The term “absolute zero” refers to a) the temperature at which water freezes. b) the temperature at which carbon dioxide freezes. c) the zero point in the Fahrenheit scale. d) the temperature at which all particle motion stops. e) the coldest temperature ever achieved on Earth. ...
Greenhouse versus living room model
... already during 150 years equal to 0,05 °C with every increase of 1 TeraWatt (~30 ExaJoule/year) worldwide generated power. This article describes the thermo dynamical research regarding the possibility of a ...
... already during 150 years equal to 0,05 °C with every increase of 1 TeraWatt (~30 ExaJoule/year) worldwide generated power. This article describes the thermo dynamical research regarding the possibility of a ...
W9e „Heat Capacity of Solids and Liquids“
... General. In case of a constant electrical power Pel and under the condition that the temperature rise above room temperature is not too large, the temperature rises linearly in a time interval t. The energy supplied by the electrical heater is Q Ctot T Pel t . This is absorbed in form of hea ...
... General. In case of a constant electrical power Pel and under the condition that the temperature rise above room temperature is not too large, the temperature rises linearly in a time interval t. The energy supplied by the electrical heater is Q Ctot T Pel t . This is absorbed in form of hea ...
Thermodynamics test
... b) if Object A has a higher temperature than Object B, Object A must have more thermal energy than Object B. c) it has the same meaning as work d) it is related to degree of hotness 12) The specific heat capacity of a substance is the heat required to warm a) it by 1 degree Celsius b) 1 kilogram of ...
... b) if Object A has a higher temperature than Object B, Object A must have more thermal energy than Object B. c) it has the same meaning as work d) it is related to degree of hotness 12) The specific heat capacity of a substance is the heat required to warm a) it by 1 degree Celsius b) 1 kilogram of ...
Mechanical Engineering 2007 Papers
... (A) 46.40% (B) 56.10% (C) 58.20% (D) 62.80% Q.35 A building has to be maintained at 21°C (dry bulb) and 14.SoC (wet bulb). The dew point temperature under these conditions is 10.l7°C. The outside temperature is -23°C (dry bulb) and the internal and external surface heat transfer coefficients are 8 W ...
... (A) 46.40% (B) 56.10% (C) 58.20% (D) 62.80% Q.35 A building has to be maintained at 21°C (dry bulb) and 14.SoC (wet bulb). The dew point temperature under these conditions is 10.l7°C. The outside temperature is -23°C (dry bulb) and the internal and external surface heat transfer coefficients are 8 W ...
12.1 Thermodynamic Systems, States, and Processes 12.3
... MC There is no heat flow into or out of the system in an (a) isothermal process, (b) adiabatic process, (c) isobaric process, (d) isometric process. MC According to the first law of thermodynamics, if work is done on a system, then (a) the internal energy of the system must change, (b) heat must be ...
... MC There is no heat flow into or out of the system in an (a) isothermal process, (b) adiabatic process, (c) isobaric process, (d) isometric process. MC According to the first law of thermodynamics, if work is done on a system, then (a) the internal energy of the system must change, (b) heat must be ...
Specific Heat of a Metal
... temperature. The heat gained by the cooler substance equals the heat lost by the warmer substance, if we assume no loss of heat to the surrounding environment. Heat lost = Heat gained In this experiment, you will determine the specific heat constant of a metal. The metal sample will be heated to a h ...
... temperature. The heat gained by the cooler substance equals the heat lost by the warmer substance, if we assume no loss of heat to the surrounding environment. Heat lost = Heat gained In this experiment, you will determine the specific heat constant of a metal. The metal sample will be heated to a h ...
Thermosphere EUV absorption Thermal Conductivity Mesopause
... dFs " cp = µ : reduction in the energy flux dt dz assumes unit efficiency of photon energy to heat dT dFs " cp = µ = # absn abs Fs dt dz ________________________________________ ...
... dFs " cp = µ : reduction in the energy flux dt dz assumes unit efficiency of photon energy to heat dT dFs " cp = µ = # absn abs Fs dt dz ________________________________________ ...
Thermal Comfort
... •Average of the air temperature of a space and the average of the various surface temperatures surrounding the space ...
... •Average of the air temperature of a space and the average of the various surface temperatures surrounding the space ...
193008 - Thermal Hydraulics
... 10. A reactor coolant system natural circulation cooldown is in progress with steam release from the steam generator (SG) atmospheric steam relief valves (operated in manual control). If high point voiding interrupts natural circulation, which one of the following will occur? (Assume feedwater flow ...
... 10. A reactor coolant system natural circulation cooldown is in progress with steam release from the steam generator (SG) atmospheric steam relief valves (operated in manual control). If high point voiding interrupts natural circulation, which one of the following will occur? (Assume feedwater flow ...
Thermochemistry - Lincoln
... transform a given amount of heat completely into work. 12.11.68 Recount the concept of entropy and know that entropy in the universe considered as a whole always increases. National Standards NSES B5a The total energy of the universe is constant. Energy can be transferred by collisions in chemical a ...
... transform a given amount of heat completely into work. 12.11.68 Recount the concept of entropy and know that entropy in the universe considered as a whole always increases. National Standards NSES B5a The total energy of the universe is constant. Energy can be transferred by collisions in chemical a ...
Name: Date: ______ Thermochemistry Round Robin
... 13. The specific heat of silver is 0.235 J/g˚C. Its melting point is 962˚C, and its heat of fusion is 11.3 kJ/mol. What quantity of heat, in joules, is required to change 5.00 g of silver from solid at 25˚C to liquid at 962˚C? ...
... 13. The specific heat of silver is 0.235 J/g˚C. Its melting point is 962˚C, and its heat of fusion is 11.3 kJ/mol. What quantity of heat, in joules, is required to change 5.00 g of silver from solid at 25˚C to liquid at 962˚C? ...
Round LED Module Thermal Management
... Ts meas is the measured LED temperature at the location in Figure 4 (⁰C) Ta avg spec is the expected average system ambient temperature (⁰C) Ta meas is the measured system ambient temperature (⁰C) The testing environment of the LED module and cooling system should simulate the application environmen ...
... Ts meas is the measured LED temperature at the location in Figure 4 (⁰C) Ta avg spec is the expected average system ambient temperature (⁰C) Ta meas is the measured system ambient temperature (⁰C) The testing environment of the LED module and cooling system should simulate the application environmen ...
Glossary of Terms - NJR Home Services
... they are pumping out too much heat/cooling and reach the thermostat’s setting too quickly), which can shorten the life of the unit dramatically. ...
... they are pumping out too much heat/cooling and reach the thermostat’s setting too quickly), which can shorten the life of the unit dramatically. ...
Apparatus to measure high-temperature thermal conductivity and
... details of instrument fabrication, the method of calibration, and typical measurements on test samples are described. The apparatus can also be used to measure the Seebeck coefficient in the same temperature range. As an example we report the thermal properties of CrSi2, which is a potential candida ...
... details of instrument fabrication, the method of calibration, and typical measurements on test samples are described. The apparatus can also be used to measure the Seebeck coefficient in the same temperature range. As an example we report the thermal properties of CrSi2, which is a potential candida ...
Chemistry/Physical Science - Thermodynamics
... section A; K is thermal conductivity; Δt is change in temp; and ΔT is change in time b. K is heat in kcal that will pass in 1 sec through a 1m3 with 2 opoosite sides w/ a 1oC difference in temperature c. R value (1) measure of thermal conductivity (2) Heat flow over time = thermal conductivity x are ...
... section A; K is thermal conductivity; Δt is change in temp; and ΔT is change in time b. K is heat in kcal that will pass in 1 sec through a 1m3 with 2 opoosite sides w/ a 1oC difference in temperature c. R value (1) measure of thermal conductivity (2) Heat flow over time = thermal conductivity x are ...
Classification of matter: Properties
... against the skin. This water is then warmed by body heat and acts as an insulator. Wetsuits are made out of closed-cell, foam neoprene. Neoprene is a synthetic rubber that contains small bubbles of nitrogen gas when made for use as wetsuit material. Nitrogen gas has very low thermal conductivity, so ...
... against the skin. This water is then warmed by body heat and acts as an insulator. Wetsuits are made out of closed-cell, foam neoprene. Neoprene is a synthetic rubber that contains small bubbles of nitrogen gas when made for use as wetsuit material. Nitrogen gas has very low thermal conductivity, so ...
Thermochemistry - hrsbstaff.ednet.ns.ca
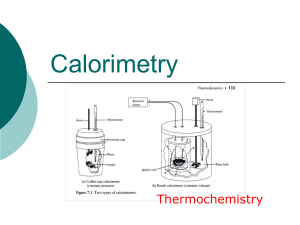
... Note: When a styrene (Styrofoam) cup is used, often it is ignored in the calculations. It is assumed that the heat absorbed by the cup is negligible. If the heat absorbed by the cup should be included in the calculations, information about the heat capacity or specific heat capacity will be given in ...
... Note: When a styrene (Styrofoam) cup is used, often it is ignored in the calculations. It is assumed that the heat absorbed by the cup is negligible. If the heat absorbed by the cup should be included in the calculations, information about the heat capacity or specific heat capacity will be given in ...