2.1c Notes - Vanderbilt University
... This results in the maxima observed in the cosmic element abundances at certain mass numbers (see slide 6 in Section 2.1c). The deduced r-process abundance distribution is, therefore, one of Nature’s signatures for the existence of shell structure in very neutron-rich nuclei. In particular, the theo ...
... This results in the maxima observed in the cosmic element abundances at certain mass numbers (see slide 6 in Section 2.1c). The deduced r-process abundance distribution is, therefore, one of Nature’s signatures for the existence of shell structure in very neutron-rich nuclei. In particular, the theo ...
Notes: Nuclear Chemistry
... a. Stability of the nucleus depends on the ratio between the # of protons and the # of neutrons (too many or too few neutrons). a. For light elements (1-20), proton to neutron ratio should be 1:1 for stable nuclei (non-radioactive). a. For heavier elements (above 20), proton to neutron ratio increas ...
... a. Stability of the nucleus depends on the ratio between the # of protons and the # of neutrons (too many or too few neutrons). a. For light elements (1-20), proton to neutron ratio should be 1:1 for stable nuclei (non-radioactive). a. For heavier elements (above 20), proton to neutron ratio increas ...
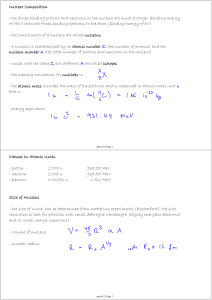
Masses in Atomic Units - proton 1.007 u 938.28 MeV
... of MeV) than the forces binding electrons to the atom (binding energy of eV) - the constituents of a nucleus are called nucleons - a nucleus is characterized by its atomic number Z (the number of protons) and the nucleon number A (the total number of protons and neutrons in the nucleus) - nuclei wit ...
... of MeV) than the forces binding electrons to the atom (binding energy of eV) - the constituents of a nucleus are called nucleons - a nucleus is characterized by its atomic number Z (the number of protons) and the nucleon number A (the total number of protons and neutrons in the nucleus) - nuclei wit ...
Nuclear Stability
... • All nuclides with 84 or more protons are unstable with respect to radio active decay. • Light nuclides are stable when neutron/proton = 1. For heavier elements the neutron /proton ratio required for stability is greater than 1 and increases with Z. • Nuclides with even numbers of protons and neutr ...
... • All nuclides with 84 or more protons are unstable with respect to radio active decay. • Light nuclides are stable when neutron/proton = 1. For heavier elements the neutron /proton ratio required for stability is greater than 1 and increases with Z. • Nuclides with even numbers of protons and neutr ...
L37 - University of Iowa Physics
... • As soon as a living organism dies, it stops taking in new carbon. The ratio of carbon-12 to carbon14 at the moment of death is the same as every other living thing, but the carbon-14 decays and is not replaced. The carbon-14 decays with its halflife of 5,700 years, while the amount of carbon-12 re ...
... • As soon as a living organism dies, it stops taking in new carbon. The ratio of carbon-12 to carbon14 at the moment of death is the same as every other living thing, but the carbon-14 decays and is not replaced. The carbon-14 decays with its halflife of 5,700 years, while the amount of carbon-12 re ...
Review and Radioactivity
... reduced by 2 and its mass number reduced by 4 (that is, by 2 protons and 2 neutrons). ...
... reduced by 2 and its mass number reduced by 4 (that is, by 2 protons and 2 neutrons). ...
Multiple Choice Questions
... (3) unstable nuclei emit alpha particles (4) unstable nuclei emit beta particles 8. Alpha particles are emitted during the radioactive decay of (1) carbon-14 (2) neon-19 (3) calcium-37 ...
... (3) unstable nuclei emit alpha particles (4) unstable nuclei emit beta particles 8. Alpha particles are emitted during the radioactive decay of (1) carbon-14 (2) neon-19 (3) calcium-37 ...
Radioactivity Unit - hrsbstaff.ednet.ns.ca
... 5. In #3 and #4 above, which reactions involved the emission of a neutrino? Which involved the emission of an antineutrino? 6. Write the equation for the electron capture of chromium 51. 7. The mass of a Beryllium 7 nucleus is 1.1652 10-26 kg. a. What is the difference between the mass of the nucl ...
... 5. In #3 and #4 above, which reactions involved the emission of a neutrino? Which involved the emission of an antineutrino? 6. Write the equation for the electron capture of chromium 51. 7. The mass of a Beryllium 7 nucleus is 1.1652 10-26 kg. a. What is the difference between the mass of the nucl ...
Quantum Physics and Nuclear Physics
... in an excited state, elevated to a higher nuclear energy level. It can become more stable by emitting a gamma particle and thus falling to an energy level closer to its ground state. ...
... in an excited state, elevated to a higher nuclear energy level. It can become more stable by emitting a gamma particle and thus falling to an energy level closer to its ground state. ...
Unit 3 – Atomic Structure
... • Isotope -Atoms of the same element with different numbers of neutrons • Mass number -The total number of protons and neutrons in a nucleus • Subatomic particles -The three kinds of particles that make up atoms: protons, neutrons, and electrons. • Nuclear fission - Splitting of the nucleus into sma ...
... • Isotope -Atoms of the same element with different numbers of neutrons • Mass number -The total number of protons and neutrons in a nucleus • Subatomic particles -The three kinds of particles that make up atoms: protons, neutrons, and electrons. • Nuclear fission - Splitting of the nucleus into sma ...
Nuclear Chemistry
... Emits electromagnetic radiation Nuclear radiation—radiation emitted from nucleus ...
... Emits electromagnetic radiation Nuclear radiation—radiation emitted from nucleus ...
Honors Question – Black Holes and Neutron Stars In Friday`s lecture
... neutron-only nucleus. It is held to a certain size by the Pauli Exclusion Principle of quantum mechanics, which says that two identical neutrons cannot occupy the same space. The theory of neutron stars was developed years ago, and the result is that they have a very specific size and mass. The radi ...
... neutron-only nucleus. It is held to a certain size by the Pauli Exclusion Principle of quantum mechanics, which says that two identical neutrons cannot occupy the same space. The theory of neutron stars was developed years ago, and the result is that they have a very specific size and mass. The radi ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.