File
... and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up the nucleus. This mass difference is called the mass defect or mass deficiency, next, Einstein's formula: E = mc2 can be u ...
... and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up the nucleus. This mass difference is called the mass defect or mass deficiency, next, Einstein's formula: E = mc2 can be u ...
Natural Radioactivity
... The strong force acts only between neutrons and protons and is responsible for -decay. The weak force acts between all known particles with a rest mass. Thus, we know that neutron decay must intermediated by the weak force, because the strong force cannot create electrons or neutrinos. All -decays ...
... The strong force acts only between neutrons and protons and is responsible for -decay. The weak force acts between all known particles with a rest mass. Thus, we know that neutron decay must intermediated by the weak force, because the strong force cannot create electrons or neutrinos. All -decays ...
Radioactivity
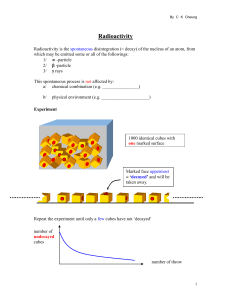
... Radioactivity is the spontaneous disintegration (= decay) of the nucleus of an atom, from which may be emitted some or all of the followings: 1/ -particle 2/ -particle 3/ rays This spontaneous process is not affected by: a/ chemical combination (e.g. ________________) b/ ...
... Radioactivity is the spontaneous disintegration (= decay) of the nucleus of an atom, from which may be emitted some or all of the followings: 1/ -particle 2/ -particle 3/ rays This spontaneous process is not affected by: a/ chemical combination (e.g. ________________) b/ ...
Atomic Nuclei - RAJEEV Classes
... short distance of about 2 – 3 fm of separation between any two nucleons. c)The nuclear force does not depend on the charge of the nucleon. ...
... short distance of about 2 – 3 fm of separation between any two nucleons. c)The nuclear force does not depend on the charge of the nucleon. ...
The nucleus
... B = (mass of nucleons) - (mass of nucleus) e.g. m n + m p – m D = B > 0 the potential energy of the nucleus is negative m n + m p + ( – E int ) = m D so B = E int the binding energy per nucleon B/A varies with A and is in the interval 5 MeV - 10 MeV (Krane) a A>62: B/A decreases with A : fission a A ...
... B = (mass of nucleons) - (mass of nucleus) e.g. m n + m p – m D = B > 0 the potential energy of the nucleus is negative m n + m p + ( – E int ) = m D so B = E int the binding energy per nucleon B/A varies with A and is in the interval 5 MeV - 10 MeV (Krane) a A>62: B/A decreases with A : fission a A ...
Nuclear Reactions Radioactive Decay The stability of an isotope
... materials, including plutonium taken from old nuclear weapons, and used fuel rods from other nuclear plant designs. CANDU reactors use heavy water (D2O – where D = 2H) to slow down neutrons so they can be more easily absorbed by the radioactive isotopes to initiate nuclear fission. ...
... materials, including plutonium taken from old nuclear weapons, and used fuel rods from other nuclear plant designs. CANDU reactors use heavy water (D2O – where D = 2H) to slow down neutrons so they can be more easily absorbed by the radioactive isotopes to initiate nuclear fission. ...
Nuclear Physics
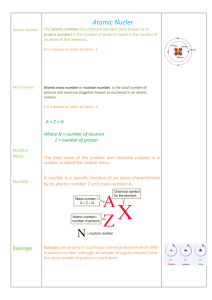
... The atomic number Z of an element is equal to the number of protons in the nucleus of that element. The mass number A of an element is equal to the total number of nucleons (protons + neutrons). The mass number A of any element is equal to the sum of the atomic number Z and the number of neutrons N ...
... The atomic number Z of an element is equal to the number of protons in the nucleus of that element. The mass number A of an element is equal to the total number of nucleons (protons + neutrons). The mass number A of any element is equal to the sum of the atomic number Z and the number of neutrons N ...
Radioactivity
... • If there are too many protons in a nucleus, it may capture an electron • A proton becomes a neutron ...
... • If there are too many protons in a nucleus, it may capture an electron • A proton becomes a neutron ...
- Physics
... Isotopes that have too few neutrons are candidates for the inverse beta decay. page 8 Alpha Particles If you recall, Rutherford used alpha particles to probe the size of the nucleus. The alpha particles are helium nuclei, 2 protons and 2 neutrons. This package of nucleons is very stable and appears ...
... Isotopes that have too few neutrons are candidates for the inverse beta decay. page 8 Alpha Particles If you recall, Rutherford used alpha particles to probe the size of the nucleus. The alpha particles are helium nuclei, 2 protons and 2 neutrons. This package of nucleons is very stable and appears ...
Chapter 29: Nuclear Physics
... Like an atom, a nucleus can be put into an excited state if it absorbs a photon of the correct energy. The nucleus can then emit a photon to go to a lower energy state. ...
... Like an atom, a nucleus can be put into an excited state if it absorbs a photon of the correct energy. The nucleus can then emit a photon to go to a lower energy state. ...
nuclear chemistry - Magoffin County Schools
... • For elements 1 – 20, need equal numbers of P+ and No. • For elements 21 – 83, increasingly more and more No than P+ are needed. • Beyond element 83, there are no stable atoms!!! ...
... • For elements 1 – 20, need equal numbers of P+ and No. • For elements 21 – 83, increasingly more and more No than P+ are needed. • Beyond element 83, there are no stable atoms!!! ...
Nuclear Reactions Review
... The isotopes of hydrogen that are commonly used in fusion are a.hydrogen and deuterium c.tritium and deuterium b.hydrogen and tritium d.none of the above ...
... The isotopes of hydrogen that are commonly used in fusion are a.hydrogen and deuterium c.tritium and deuterium b.hydrogen and tritium d.none of the above ...
Atomic and Nuclear Physics
... • Strong, short-range nuclear interaction between nucleons (p and n) binds the nucleons • Overall balance must be correct and more neutrons needed • Strong force is very strong, short range and the same for all nucleons (as nuclei all have the same density) • Adding more neutrons (compared to proton ...
... • Strong, short-range nuclear interaction between nucleons (p and n) binds the nucleons • Overall balance must be correct and more neutrons needed • Strong force is very strong, short range and the same for all nucleons (as nuclei all have the same density) • Adding more neutrons (compared to proton ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.