Atom and Nucleus. Radioactivity. Nuclear Energy.
... Ernest Rutherford attempted to test this model by bombarding a thin gold foil with alpha-particles. A significant scattering of the alpha-particles was ...
... Ernest Rutherford attempted to test this model by bombarding a thin gold foil with alpha-particles. A significant scattering of the alpha-particles was ...
Unit_Phys_2_nuclear_fusion__fission
... that shows the life cycles of stars. Additional guidance: Candidates should be able to sketch or complete a labelled diagram to illustrate how a chain reaction may occur. ...
... that shows the life cycles of stars. Additional guidance: Candidates should be able to sketch or complete a labelled diagram to illustrate how a chain reaction may occur. ...
Homework 1
... released in Joules from burning 1 mole of ethanol to the energy released from 1 mole of 235U above. ...
... released in Joules from burning 1 mole of ethanol to the energy released from 1 mole of 235U above. ...
have shown no evidence
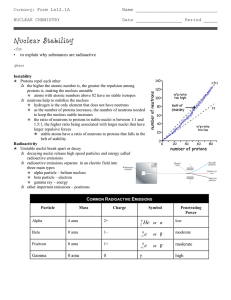
... • Stability is favoured by even numbers of protons and neutrons • Not usually equal numbers • Plotting neutron number (A) against proton number (Z) for all known nuclei, shows area of stability • For very light elements N ≈ Z gives stable elements • 1:1 up to 4020Ca • Ratio gradually rises (A>Z) unt ...
... • Stability is favoured by even numbers of protons and neutrons • Not usually equal numbers • Plotting neutron number (A) against proton number (Z) for all known nuclei, shows area of stability • For very light elements N ≈ Z gives stable elements • 1:1 up to 4020Ca • Ratio gradually rises (A>Z) unt ...
regan-kent-25nov09a
... • Gold has 79 protons (i.e. Z=79) • Start with Z=78 protons (i.e. Platinum) • Specifically 196Pt ( Pt = Z=78, N=196-78=118) • Reaction is 196Pt + neutron to make 197Pt • 197Pt is radioactive and ‘beta-decays’ to make ...
... • Gold has 79 protons (i.e. Z=79) • Start with Z=78 protons (i.e. Platinum) • Specifically 196Pt ( Pt = Z=78, N=196-78=118) • Reaction is 196Pt + neutron to make 197Pt • 197Pt is radioactive and ‘beta-decays’ to make ...
Atomic Structure and Radioactivity
... Radioactivity is associated with the nucleus and is not affected by chemical reactions or heating. ...
... Radioactivity is associated with the nucleus and is not affected by chemical reactions or heating. ...
Glossary of Key Terms in Chapter Two
... beta particle (9.1) an electron formed in the nucleus by the conversion of a neutron into a proton. binding energy (9.3) the energy required to break down the nucleus into its component parts. breeder reactor (9.4) a nuclear reactor that produces its own fuel in the process of providing electrical e ...
... beta particle (9.1) an electron formed in the nucleus by the conversion of a neutron into a proton. binding energy (9.3) the energy required to break down the nucleus into its component parts. breeder reactor (9.4) a nuclear reactor that produces its own fuel in the process of providing electrical e ...
ib atomic and nuclear physics definitions and concepts
... a nucleus. A large binding energy implies a stable nucleus. MASS DEFECT: The mass of the particles of the separated nucleus is greater than when they are combined. Mass defect = mass of parts - mass of nucleus EINSTEIN MASS-ENERGY EQUIVALENCE: E = mc2. The mass defect is equivalent to a change in ...
... a nucleus. A large binding energy implies a stable nucleus. MASS DEFECT: The mass of the particles of the separated nucleus is greater than when they are combined. Mass defect = mass of parts - mass of nucleus EINSTEIN MASS-ENERGY EQUIVALENCE: E = mc2. The mass defect is equivalent to a change in ...
IB-ATOMIC-AND-NUCLEAR-PHYSICS-DEFINITIONS
... a nucleus. A large binding energy implies a stable nucleus. MASS DEFECT: The mass of the particles of the separated nucleus is greater than when they are combined. Mass defect = mass of parts - mass of nucleus EINSTEIN MASS-ENERGY EQUIVALENCE: E = mc2. The mass defect is equivalent to a change in ...
... a nucleus. A large binding energy implies a stable nucleus. MASS DEFECT: The mass of the particles of the separated nucleus is greater than when they are combined. Mass defect = mass of parts - mass of nucleus EINSTEIN MASS-ENERGY EQUIVALENCE: E = mc2. The mass defect is equivalent to a change in ...
Chapter 25
... Unstable nuclei decay at a constant rate – cannot be affected. – In comparison to ____________ ________________. ...
... Unstable nuclei decay at a constant rate – cannot be affected. – In comparison to ____________ ________________. ...
Nuclear Reactions
... Nuclear reactions The stability of isotopes is based on the ratio of neutrons and protons in its nucleus. Although most nuclei are stable, some are unstable and spontaneously decay, emitting radiation. Each radioactive isotope has a specific mode and rate of decay (half-life). A change in the nucleu ...
... Nuclear reactions The stability of isotopes is based on the ratio of neutrons and protons in its nucleus. Although most nuclei are stable, some are unstable and spontaneously decay, emitting radiation. Each radioactive isotope has a specific mode and rate of decay (half-life). A change in the nucleu ...
nuclear chemistry notes 1
... same number of protons and neutrons. This is when they are the most stable. Large atoms with around 83 protons or more are so large that they too are unstable. If atoms are too unstable, they will decompose, or radioactively decay, releasing different types of particles in order to become more stabl ...
... same number of protons and neutrons. This is when they are the most stable. Large atoms with around 83 protons or more are so large that they too are unstable. If atoms are too unstable, they will decompose, or radioactively decay, releasing different types of particles in order to become more stabl ...
SIMPLE NUCLEAR REACTIONS
... nucleus within an atom. There are three main types of decay: Alpha Decay () An alpha particle contains 2 protons, and 2 neutrons so it is often referred to as a helium nucleus. Radium-226 is an emitter. Ex. 226 ...
... nucleus within an atom. There are three main types of decay: Alpha Decay () An alpha particle contains 2 protons, and 2 neutrons so it is often referred to as a helium nucleus. Radium-226 is an emitter. Ex. 226 ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.