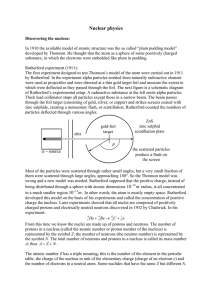
View - Rutgers Physics
... formula might say, and those with an odd number a bit less well bound. This has a considerable effect on which nuclei are stable; If you look closely at the stable nuclei in Fig. 44.3, you see many more blue dots for even values of N and Z then for the odd values. Secondly, as you can imagine, the e ...
... formula might say, and those with an odd number a bit less well bound. This has a considerable effect on which nuclei are stable; If you look closely at the stable nuclei in Fig. 44.3, you see many more blue dots for even values of N and Z then for the odd values. Secondly, as you can imagine, the e ...
Phys214 Final Exam
... 12. Consider two different samples of radioactive isotopes, one naturally occurring and the other artificially produced. If the samples have the same number of nuclei, then A. the one with the shorter half-life is likely more dangerous. B. the one with the smaller atomic mass is likely more dangerou ...
... 12. Consider two different samples of radioactive isotopes, one naturally occurring and the other artificially produced. If the samples have the same number of nuclei, then A. the one with the shorter half-life is likely more dangerous. B. the one with the smaller atomic mass is likely more dangerou ...
Atomic and Nuclear Terms
... ► γ - Gamma Decay – Process in which a nucleus in an excited state drops to a lower energy state emitting gamma particle. ...
... ► γ - Gamma Decay – Process in which a nucleus in an excited state drops to a lower energy state emitting gamma particle. ...
Nuclear Chemistry PowerPoint presentation
... levels, or shells, in the nucleus. The number of nucleons that represent completed nuclear energy levels, ...
... levels, or shells, in the nucleus. The number of nucleons that represent completed nuclear energy levels, ...
Nuclear physics α −
... molecular bonds and disturb the biochemical reactions. From now under radiation we include radioactivity (alpha, beta, and gamma radiations), neutrons and X-rays. Charged particles ...
... molecular bonds and disturb the biochemical reactions. From now under radiation we include radioactivity (alpha, beta, and gamma radiations), neutrons and X-rays. Charged particles ...
Syllabus overview
... 3.1.1 State that temperature determines the direction of thermal energy transfer between two objects. Students should be familiar with the concept of thermal equilibrium. 3.1.2 State the relation between the Kelvin and Celsius scales of temperature. T/K = t/°C + 273 is sufficient. 3.1.3 State that t ...
... 3.1.1 State that temperature determines the direction of thermal energy transfer between two objects. Students should be familiar with the concept of thermal equilibrium. 3.1.2 State the relation between the Kelvin and Celsius scales of temperature. T/K = t/°C + 273 is sufficient. 3.1.3 State that t ...
A – Z - washburnsciencelies
... to form a single larger nuclei. This produces far more energy than a fission reaction, and also does not have a dangerous by-product. However we currently don’t have the means to use it as a reliable energy source, as we barely get more energy out, than we put in. ...
... to form a single larger nuclei. This produces far more energy than a fission reaction, and also does not have a dangerous by-product. However we currently don’t have the means to use it as a reliable energy source, as we barely get more energy out, than we put in. ...
Nuclear Reactions - Socastee High School
... in red giants and supernovae is that in supernovae the flux of neutrons is greater and it is possible for the atom to capture a second, or third neutron, before it has a chance to beta-decay. • This leads to the production of a different set of elements to those produced in red giants, where the flu ...
... in red giants and supernovae is that in supernovae the flux of neutrons is greater and it is possible for the atom to capture a second, or third neutron, before it has a chance to beta-decay. • This leads to the production of a different set of elements to those produced in red giants, where the flu ...
Nuclear Binding Energy
... will yield nuclei which are more tightly bound (less mass per nucleon). The binding energies of nucleons are in the range of millions of electron volts compared to tens of eV for atomic electrons. Whereas an atomic transition might emit a photon in the range of a few electron volts, perhaps in the v ...
... will yield nuclei which are more tightly bound (less mass per nucleon). The binding energies of nucleons are in the range of millions of electron volts compared to tens of eV for atomic electrons. Whereas an atomic transition might emit a photon in the range of a few electron volts, perhaps in the v ...
nuclear physics nuclear physics
... atomic bombs, such as uranium bombs (like the one that hit Hiroshima) or plutonium bombs (like the one that hit Nagasaki). In nuclear fission, when a core of fissile material (which produces fission neutrons with any kinetic energy) or fissionable (only with high kinetic energy of neutrons, called f ...
... atomic bombs, such as uranium bombs (like the one that hit Hiroshima) or plutonium bombs (like the one that hit Nagasaki). In nuclear fission, when a core of fissile material (which produces fission neutrons with any kinetic energy) or fissionable (only with high kinetic energy of neutrons, called f ...
Alpha Decay
... gamma rays have the same basic properties but come from different parts of the atom. X-rays are emitted from processes outside the nucleus, but gamma rays originate inside the nucleus. They also are generally lower in energy and, therefore, less penetrating than gamma rays. X-rays can be produced na ...
... gamma rays have the same basic properties but come from different parts of the atom. X-rays are emitted from processes outside the nucleus, but gamma rays originate inside the nucleus. They also are generally lower in energy and, therefore, less penetrating than gamma rays. X-rays can be produced na ...
MAJOR NUCLEAR BURNING STAGES The Coulomb barrier is
... Three general principles influence the roles that these nuclear burning stages may play: 1.Successive nuclear burning stages, involving more massive nuclei with higher charges, will require increasingly high temperatures to overcome the increased electrical repulsion. 2.The amount of energy released ...
... Three general principles influence the roles that these nuclear burning stages may play: 1.Successive nuclear burning stages, involving more massive nuclei with higher charges, will require increasingly high temperatures to overcome the increased electrical repulsion. 2.The amount of energy released ...
Nuclear Radiation1516
... several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent of the original mass) has been converted ...
... several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent of the original mass) has been converted ...
Atomic Structure: 1. The smallest particle of an element that retains
... Cobalt–60 has a half–life of 5.25 years. How much of the substance would remain after two half–lives? ...
... Cobalt–60 has a half–life of 5.25 years. How much of the substance would remain after two half–lives? ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.