Chapter39
... A nucleon is a general term to denote a nuclear particle - that is, either a proton or a neutron. The atomic number Z of an element is equal to the number of protons in the nucleus of that element. The mass number A of an element is equal to the total number of nucleons (protons + neutrons). The mas ...
... A nucleon is a general term to denote a nuclear particle - that is, either a proton or a neutron. The atomic number Z of an element is equal to the number of protons in the nucleus of that element. The mass number A of an element is equal to the total number of nucleons (protons + neutrons). The mas ...
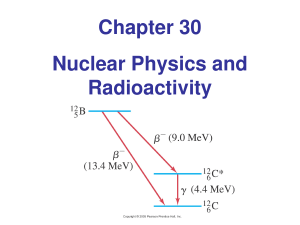
Chapter 30 Nuclear Physics and Radioactivity
... living plant or tree will be constantly exchanging carbon with the atmosphere, and will have the same carbon ratio in its tissues. ...
... living plant or tree will be constantly exchanging carbon with the atmosphere, and will have the same carbon ratio in its tissues. ...
nuclear reactions
... Fusion is the process that powers active stars. • Fusion reactions have the greatest energy density, that is energy per unit of mass, than any known process (nuclear fission or chemical reactions). ...
... Fusion is the process that powers active stars. • Fusion reactions have the greatest energy density, that is energy per unit of mass, than any known process (nuclear fission or chemical reactions). ...
Inside the Atom connections to the GCSE (KS4) curriculum for England
... - Rutherford’s discovery of the nucleus, leading to the birth of nuclear physics. - How proton and neutron number defines elements and isotopes, the basis of explaining the nuclear chart. - Radioactivity and ionising radiation, these topics provide great opportunities for practical physics – e ...
... - Rutherford’s discovery of the nucleus, leading to the birth of nuclear physics. - How proton and neutron number defines elements and isotopes, the basis of explaining the nuclear chart. - Radioactivity and ionising radiation, these topics provide great opportunities for practical physics – e ...
Jan. 11: Introduction
... 1896: discovery of radioactivity by Becquerel 1898: separation of Radium by Maria and Pierre Curie; discovery of α, β, γ rays 1911: nucleus as a central part of an atom – Rutherford 1913: Soddy and Richards elucidate the concept of nuclear mass: isotopes are born 1917: Rutherford carries out first t ...
... 1896: discovery of radioactivity by Becquerel 1898: separation of Radium by Maria and Pierre Curie; discovery of α, β, γ rays 1911: nucleus as a central part of an atom – Rutherford 1913: Soddy and Richards elucidate the concept of nuclear mass: isotopes are born 1917: Rutherford carries out first t ...
chap7_nucleus
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
Particle Physics at Soudan - Soudan Underground Laboratory
... Which three quarks make up the proton (charge +e)? Answer:_____ _____ _____ Which three quarks make up the neutron (zero charge)? Answer:_____ _____ _____ The electron is not made of quarks, but is a fundamental (indivisible) particle called a lepton. It’s a lot smaller than a proton or neutron, wit ...
... Which three quarks make up the proton (charge +e)? Answer:_____ _____ _____ Which three quarks make up the neutron (zero charge)? Answer:_____ _____ _____ The electron is not made of quarks, but is a fundamental (indivisible) particle called a lepton. It’s a lot smaller than a proton or neutron, wit ...
Particle Physics at Soudan These questions will help you prepare
... Which three quarks make up the proton (charge +e)? Answer:_____ _____ _____ Which three quarks make up the neutron (zero charge)? Answer:_____ _____ _____ The electron is not made of quarks, but is a fundamental (indivisible) particle called a lepton. It’s a lot smaller than a proton or neutron, wit ...
... Which three quarks make up the proton (charge +e)? Answer:_____ _____ _____ Which three quarks make up the neutron (zero charge)? Answer:_____ _____ _____ The electron is not made of quarks, but is a fundamental (indivisible) particle called a lepton. It’s a lot smaller than a proton or neutron, wit ...
Nuclear Physics and Radioactivity2
... charge is q=0; its mass is mn = 1.6749 x 10-27 kg. Chadwick's discovery established that the nucleus contained two types of particles -- protons and neutrons. Nucleons The term that refers to the two constituent particles of a nucleus (protons and neutrons). Atomic Number The number of protons in a ...
... charge is q=0; its mass is mn = 1.6749 x 10-27 kg. Chadwick's discovery established that the nucleus contained two types of particles -- protons and neutrons. Nucleons The term that refers to the two constituent particles of a nucleus (protons and neutrons). Atomic Number The number of protons in a ...
Purdue University PHYS 221 FINAL EXAM (orange) 12/17/03
... A coin is dropped into a fountain of depth 60 cm. To someone standing outside the fountain, how far below the surface of the water does the coin appear to be when it comes to rest? (The refractive index of water is 1.33.) a) b) c) d) e) ...
... A coin is dropped into a fountain of depth 60 cm. To someone standing outside the fountain, how far below the surface of the water does the coin appear to be when it comes to rest? (The refractive index of water is 1.33.) a) b) c) d) e) ...
helium
... temperature, T, don’t depend on what happened at earlier times and higher temperature ...
... temperature, T, don’t depend on what happened at earlier times and higher temperature ...
CH_8_nucleus_new
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
CHAPTER 4: ABUNDANCE AND RADIOACTIVITY OF UNSTABLE
... distribution is such that the maximum in this distribution is at an energy about one third of the maximum energy. As mentioned above, in some cases the resulting daughter nucleus is not in an excited state, so that no g radiation is emitted. This happens to be the case with the two isotopes that are ...
... distribution is such that the maximum in this distribution is at an energy about one third of the maximum energy. As mentioned above, in some cases the resulting daughter nucleus is not in an excited state, so that no g radiation is emitted. This happens to be the case with the two isotopes that are ...
Sample pages 1 PDF
... corresponds to the Weizsäcker mass formula (2.8). Nuclei with a small number of nucleons display relatively large deviations from the general trend, and should be considered on an individual basis. For heavy nuclei deviations in the form of a somewhat stronger binding per nucleon are also observed f ...
... corresponds to the Weizsäcker mass formula (2.8). Nuclei with a small number of nucleons display relatively large deviations from the general trend, and should be considered on an individual basis. For heavy nuclei deviations in the form of a somewhat stronger binding per nucleon are also observed f ...
Nuclear and Particle Physics - Lecture 26 Nucleosynthesis 1
... too hot to form nuclei; the collisions were just too energetic for any nuclear binding to survive. The matter was all separate particles, namely electrons, neutrinos, protons and neutrons; the latter have a lifetime of around 15 minutes and so had not decayed significantly yet. There were also a lot ...
... too hot to form nuclei; the collisions were just too energetic for any nuclear binding to survive. The matter was all separate particles, namely electrons, neutrinos, protons and neutrons; the latter have a lifetime of around 15 minutes and so had not decayed significantly yet. There were also a lot ...
Name: Period: ______ Date: Fission and Fusion Simulations Fission
... 6. Reset All. Toggle so there is 1 single nucleus of U-238. Press the red button to shoot a neutron into it and describe what happens. ...
... 6. Reset All. Toggle so there is 1 single nucleus of U-238. Press the red button to shoot a neutron into it and describe what happens. ...
E = mc2 (Einstein)
... poker game. We can understand the energy minimization principle from daily life as well. For example, we would rather sit than stand; this is because by sitting we minimize our (gravitational) potential energy. Stable isotopes are simply those that have attained this ...
... poker game. We can understand the energy minimization principle from daily life as well. For example, we would rather sit than stand; this is because by sitting we minimize our (gravitational) potential energy. Stable isotopes are simply those that have attained this ...
Nuclear and Particle Physics SubAtomic Physics
... Oldest rocks measured to date: ~ 4.5 billion years old ! ...
... Oldest rocks measured to date: ~ 4.5 billion years old ! ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.