The science of chemistry is concerned with the composition
... Neither, for that matter, did the man whose experiments and ideas led directly to the theory itself. Antoine Lavoisier was born in 1743, the same year as Thomas Jefferson. The son of a wealthy French lawyer, he was well educated and became a successful businessman, gentleman farmer, economist, and s ...
... Neither, for that matter, did the man whose experiments and ideas led directly to the theory itself. Antoine Lavoisier was born in 1743, the same year as Thomas Jefferson. The son of a wealthy French lawyer, he was well educated and became a successful businessman, gentleman farmer, economist, and s ...
The science of chemistry is concerned with the
... Neither, for that matter, did the man whose experiments and ideas led directly to the theory itself. Antoine Lavoisier was born in 1743, the same year as Thomas Jefferson. The son of a wealthy French lawyer, he was well educated and became a successful businessman, gentleman farmer, economist, and s ...
... Neither, for that matter, did the man whose experiments and ideas led directly to the theory itself. Antoine Lavoisier was born in 1743, the same year as Thomas Jefferson. The son of a wealthy French lawyer, he was well educated and became a successful businessman, gentleman farmer, economist, and s ...
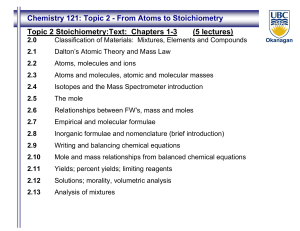
Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... ¾ Atoms can be identified by the number of protons and neutrons they contain ¾ The atomic number (Z) is the number of protons in the nucleus of each atom of an element. ¾ In a neutral atom the number of protons is equal to the number of electrons ¾ The chemical identity of an atom can be determined ...
... ¾ Atoms can be identified by the number of protons and neutrons they contain ¾ The atomic number (Z) is the number of protons in the nucleus of each atom of an element. ¾ In a neutral atom the number of protons is equal to the number of electrons ¾ The chemical identity of an atom can be determined ...
Hybridization of atomic orbitals
... learned that the electron is most likely at a distance r = 53 pm from the center of the atom. 2. Radial density (RD) versus r: To really represent the probability of finding the electron at r at a given time, the radial distribution against r is often plotted. In this plot, instead of plotting squar ...
... learned that the electron is most likely at a distance r = 53 pm from the center of the atom. 2. Radial density (RD) versus r: To really represent the probability of finding the electron at r at a given time, the radial distribution against r is often plotted. In this plot, instead of plotting squar ...
Exemplar Paper
... identify the question number you are attempting. Any rough work must be written in this booklet. You should score through your rough work when you have written your final copy. Use blue or black ink. Before leaving the examination room you must give this booklet to the Invigilator; if you do not, yo ...
... identify the question number you are attempting. Any rough work must be written in this booklet. You should score through your rough work when you have written your final copy. Use blue or black ink. Before leaving the examination room you must give this booklet to the Invigilator; if you do not, yo ...
Unit 3 2 Basic Mole Conversions and Mole Maps
... Meaning: For every 2 mol of ethane, 7 mol of dioxygen molecules are consumed in the combustion (a ratio of 2 to 7) This produces 4 moles of carbon dioxide, 6 moles of water and releases 3,170 kJ of energy from the bonding chemicals to the environment. This set of relationships can indicate, at a gla ...
... Meaning: For every 2 mol of ethane, 7 mol of dioxygen molecules are consumed in the combustion (a ratio of 2 to 7) This produces 4 moles of carbon dioxide, 6 moles of water and releases 3,170 kJ of energy from the bonding chemicals to the environment. This set of relationships can indicate, at a gla ...
CH 151 Companion
... should be long enough to tuck inside your pants. Cotton t-shirts are fine. Tank tops, scooped neck tops, leotards, sleeveless blouses and tops made of sheer material are not allowed. c. Pants and skirts must be at least knee length. d. Shoes must be flat-soled and cover the entire foot. Socks must b ...
... should be long enough to tuck inside your pants. Cotton t-shirts are fine. Tank tops, scooped neck tops, leotards, sleeveless blouses and tops made of sheer material are not allowed. c. Pants and skirts must be at least knee length. d. Shoes must be flat-soled and cover the entire foot. Socks must b ...
Default Normal Template
... E.x: Calculate the number of carbon atoms and the number of hydrogen atoms in 600g of propane, C3 H8 C = 12 , H = 1. MW = ( 3 x 12 ) + ( 8 x 1 ) = 44 amu Number of moles of propane = 600 g x 1 mol = 13.63 mol ...
... E.x: Calculate the number of carbon atoms and the number of hydrogen atoms in 600g of propane, C3 H8 C = 12 , H = 1. MW = ( 3 x 12 ) + ( 8 x 1 ) = 44 amu Number of moles of propane = 600 g x 1 mol = 13.63 mol ...
Gases - chemmybear.com
... It has the largest size or volume. It has the strongest attractive forces (van der Waals forces or dipole-dipole interactions). (c) High temperature result in high kinetic energies. This energy overcomes the attractive forces. Low pressure increases the distance between molecules. (So molecules comp ...
... It has the largest size or volume. It has the strongest attractive forces (van der Waals forces or dipole-dipole interactions). (c) High temperature result in high kinetic energies. This energy overcomes the attractive forces. Low pressure increases the distance between molecules. (So molecules comp ...
Powerpoint
... Chemistry is Everywhere! • Introduction: Everything we do, from digesting our food to making art, involves chemistry. Everything is made of chemicals! Today, we are going to learn about how chemistry is involved in tie dying. First we need to understand a few things about dyes and how they react. ...
... Chemistry is Everywhere! • Introduction: Everything we do, from digesting our food to making art, involves chemistry. Everything is made of chemicals! Today, we are going to learn about how chemistry is involved in tie dying. First we need to understand a few things about dyes and how they react. ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... Copyright © 2011 Pearson Education, Inc. ...
... Copyright © 2011 Pearson Education, Inc. ...
Gr. 11 Chemistry Student Workbook (Spring 2016)
... An active science program presents some hazards to both staff and students. All attempts will be made however, to identify hazards and manage risks so that they become minimal. Before each activity, instructions will be given to reduce any risks. Teachers will assess the readiness level of students ...
... An active science program presents some hazards to both staff and students. All attempts will be made however, to identify hazards and manage risks so that they become minimal. Before each activity, instructions will be given to reduce any risks. Teachers will assess the readiness level of students ...
Chapter 4
... 2. Ions of one atom: oxidation number = charge 3. Oxygen - usually -2; in peroxides (H2O2), O = -1 4. Hydrogen is usually +1, except when bonded to metals, e.g., LiH, NaH in which H = -1 ...
... 2. Ions of one atom: oxidation number = charge 3. Oxygen - usually -2; in peroxides (H2O2), O = -1 4. Hydrogen is usually +1, except when bonded to metals, e.g., LiH, NaH in which H = -1 ...
Week of Sept. 20
... phosphines ((-)-methylpropylphenylphosphine, 69%ee) demonstrated that a chiral transition metal complex could transfer chirality to a non-chiral ...
... phosphines ((-)-methylpropylphenylphosphine, 69%ee) demonstrated that a chiral transition metal complex could transfer chirality to a non-chiral ...
Chapter 14 (Kinetics) – Slides and Practice
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
Chapter
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...