Chemical Equations Chemical Reaction: Interaction between
... Individual products and reactants are separated by a plus sign Chemical Equation: A written statement using symbols and formulas to describe the changes that occur in a reaction Example: 2H2(g) + O2 (g) Æ 2H2O (l) Letter in parentheses indicates the state of the substance: gas (g), liquid (l), solid ...
... Individual products and reactants are separated by a plus sign Chemical Equation: A written statement using symbols and formulas to describe the changes that occur in a reaction Example: 2H2(g) + O2 (g) Æ 2H2O (l) Letter in parentheses indicates the state of the substance: gas (g), liquid (l), solid ...
Chemistry Challenge Problems
... he chemical properties of an element depend primarily on its number of valence electrons in its atoms. The noble gas elements, for example, all have similar chemical properties because the outermost energy levels of their atoms are completely filled. The chemical properties of ions also depend on th ...
... he chemical properties of an element depend primarily on its number of valence electrons in its atoms. The noble gas elements, for example, all have similar chemical properties because the outermost energy levels of their atoms are completely filled. The chemical properties of ions also depend on th ...
Preliminary review / Publisher`s description: This self
... 1. Establish the existence of global solutions. 2. Write down the first order necessary conditions. 3. Investigate these conditions. 4. Write down the conclusions. The main tools in Step 1 are the classical Weierstrass theorem (applied to some bounded non-empty sublevel set) and its extension to coe ...
... 1. Establish the existence of global solutions. 2. Write down the first order necessary conditions. 3. Investigate these conditions. 4. Write down the conclusions. The main tools in Step 1 are the classical Weierstrass theorem (applied to some bounded non-empty sublevel set) and its extension to coe ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... Write the balanced equation for the reaction that occurs when methanol,CH3OH(l), is burned in air. When any compound containing C, H, and O is combusted, it reacts with the O2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) CO2(g) + H2O(g) The C atoms are ...
... Write the balanced equation for the reaction that occurs when methanol,CH3OH(l), is burned in air. When any compound containing C, H, and O is combusted, it reacts with the O2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) CO2(g) + H2O(g) The C atoms are ...
The SimSoup Guide - Chris Gordon
... von Helmholtz also took the view that life on Earth arrived from elsewhere in the universe. The idea that life on Earth had an extra-terrestrial origin has a very long history. It can be traced back to the ancient Greek philosopher Anaxagoras. He claimed that the universe is made of an infinite numb ...
... von Helmholtz also took the view that life on Earth arrived from elsewhere in the universe. The idea that life on Earth had an extra-terrestrial origin has a very long history. It can be traced back to the ancient Greek philosopher Anaxagoras. He claimed that the universe is made of an infinite numb ...
2015 1Q4Mathp1
... 1-9 on individual slips of paper and put them in a second bag. Choose a number from each bag and write an addition equation using the numbers. Then solve the problem using a written method and verbal explanation. These verbal explanations ...
... 1-9 on individual slips of paper and put them in a second bag. Choose a number from each bag and write an addition equation using the numbers. Then solve the problem using a written method and verbal explanation. These verbal explanations ...
EXPERIMENT 3 – Keto-Enol Equilibrium Using NMR
... 1. Choose one of the β-diketones for study by the entire class. Prepare solutions of the chosen compound in at least four different solvents (C6D6, C6D12, CD3CN, H2O/D2O, CDCl3, acetone-d6 and/or dimethyl sulfoxide-d6) at a concentration of ~1 mM. Prepare them at least 60 minutes in advance of runni ...
... 1. Choose one of the β-diketones for study by the entire class. Prepare solutions of the chosen compound in at least four different solvents (C6D6, C6D12, CD3CN, H2O/D2O, CDCl3, acetone-d6 and/or dimethyl sulfoxide-d6) at a concentration of ~1 mM. Prepare them at least 60 minutes in advance of runni ...
Prep UK-intro.p65
... The aim of the scientific committee has been that as many as possible of the preparatory problems should take their starting point in issues of general chemical, public or environmental interest. Therefore some of the problems cover several topics from the International Chemistry Olympiad. We have a ...
... The aim of the scientific committee has been that as many as possible of the preparatory problems should take their starting point in issues of general chemical, public or environmental interest. Therefore some of the problems cover several topics from the International Chemistry Olympiad. We have a ...
Sample Exercise 20.1 Identifying Oxidizing and Reducing Agents
... Now we can use the summary in Figure 20.6 to help us describe the voltaic cell. The first half-reaction is the reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons ...
... Now we can use the summary in Figure 20.6 to help us describe the voltaic cell. The first half-reaction is the reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons ...
Chapter 6 Table of Contents
... However, to save on long-term energy and materials costs, the university offered to buy only 1 laser printer per 10 employees, with the plan to network the printers together. How many laser printers did the administration have to buy? It is rather simple to show that 26 laser printers are needed for ...
... However, to save on long-term energy and materials costs, the university offered to buy only 1 laser printer per 10 employees, with the plan to network the printers together. How many laser printers did the administration have to buy? It is rather simple to show that 26 laser printers are needed for ...
Worked out problems
... Now we can use the summary in Figure 20.6 to help us describe the voltaic cell. The first half-reaction is the reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons ...
... Now we can use the summary in Figure 20.6 to help us describe the voltaic cell. The first half-reaction is the reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons ...
Fundamentals
... charge (-1), their mass is small, ~1/2000 amu). If you want, you can imagine that the electrons “orbit” around the nucleus, like the planets around the sun. Example - Helium atom: 2 protons, 2 electrons, 2 neutrons, mass ~ 4 amu In an atom, the number of electrons is equal to the number of protons, ...
... charge (-1), their mass is small, ~1/2000 amu). If you want, you can imagine that the electrons “orbit” around the nucleus, like the planets around the sun. Example - Helium atom: 2 protons, 2 electrons, 2 neutrons, mass ~ 4 amu In an atom, the number of electrons is equal to the number of protons, ...
Mechanistic and Computational Studies of Ferroin, Simple Organic
... era of knowledge regarding non-linear chemical dynamics. Since we opt for an entire laboratory in silico, this study will primarily examine physical and electronic mechanisms at the single1 ...
... era of knowledge regarding non-linear chemical dynamics. Since we opt for an entire laboratory in silico, this study will primarily examine physical and electronic mechanisms at the single1 ...
Oxidation Numbers and Ionic Compounds
... 5. Subtract the number of electrons already used for the single bonds; two for each bond. 6. Distribute the remaining electrons in pairs around the atoms, trying to satisfy the octet rule. Assign them to the most electronegative atom first. 7. If you run out of electrons before all atoms have an oct ...
... 5. Subtract the number of electrons already used for the single bonds; two for each bond. 6. Distribute the remaining electrons in pairs around the atoms, trying to satisfy the octet rule. Assign them to the most electronegative atom first. 7. If you run out of electrons before all atoms have an oct ...
Stoichiometric Conversions
... gas, the two will combust and form carbon dioxide and water CH4 + 2O2 CO2 + 2H2O How many moles of H2O will be formed when 28.0 g of methane combusts? ...
... gas, the two will combust and form carbon dioxide and water CH4 + 2O2 CO2 + 2H2O How many moles of H2O will be formed when 28.0 g of methane combusts? ...
Document
... is the amount of product made in a chemical reaction. There are three types: 1. Actual yield- what you actually get in the lab when the chemicals are mixed 2. Theoretical yield- what the balanced equation tells should be made ...
... is the amount of product made in a chemical reaction. There are three types: 1. Actual yield- what you actually get in the lab when the chemicals are mixed 2. Theoretical yield- what the balanced equation tells should be made ...
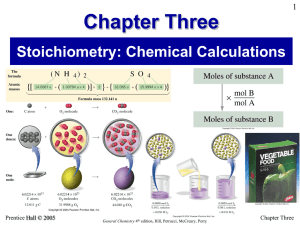
Chapter Three
... sodium, 22.67% sulfur, and 45.02% oxygen. Find the empirical formula for this compound. • HINT = Whenever you are given %s, we assume we ALWAYS have a 100-gram sample, so immediately convert you %s to grams. ...
... sodium, 22.67% sulfur, and 45.02% oxygen. Find the empirical formula for this compound. • HINT = Whenever you are given %s, we assume we ALWAYS have a 100-gram sample, so immediately convert you %s to grams. ...
Chemistry 21A: Survey of General and Organic Chemistry
... 1. use the language of general chemistry (vocabulary, nomenclature, formulas and equations) to describe chemical systems and changes (physical and chemical) they undergo. 2. describe the structure of the atom in terms of the arrangement of subatomic particles and electronic configuration. 3. extract ...
... 1. use the language of general chemistry (vocabulary, nomenclature, formulas and equations) to describe chemical systems and changes (physical and chemical) they undergo. 2. describe the structure of the atom in terms of the arrangement of subatomic particles and electronic configuration. 3. extract ...
Stoichiometry - MolesAvacado
... The following Wisconsin State Academic Standards are addressed in the lesson plans: A.12.2 Show* how conflicting assumptions about science themes* lead to different opinions and decisions about evolution*, health, population, longevity, education, and use of resources, and show* how these opinions a ...
... The following Wisconsin State Academic Standards are addressed in the lesson plans: A.12.2 Show* how conflicting assumptions about science themes* lead to different opinions and decisions about evolution*, health, population, longevity, education, and use of resources, and show* how these opinions a ...