* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Endocrine System
Glycemic index wikipedia , lookup
Mammary gland wikipedia , lookup
Endocrine disruptor wikipedia , lookup
Neuroendocrine tumor wikipedia , lookup
Growth hormone therapy wikipedia , lookup
Hypothalamus wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Adrenal gland wikipedia , lookup
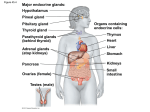
The Endocrine System Pituitary Gland Clinical manifestations of pituitary disease Pituitary adenomas and hyperpituitarism Prolactinomas Growth hormone cell (somatotroph) adenomas ACTH (corticotroph) adenomas Other anterior pituitary adenomas Hypopituitarism Posterior pituitary syndromes Hypothalmic suprasellar tumors Pituitary Gland Anterior pituitary Somatotrophs – GH Lactotrophs – prolactin Corticotrophs – ACTH, POMC, MSH Thryotrophs – TSH Gonadotrophs – FSH, LH Posterior pituitary – axonal processes from hypothalamus: Oxytocin ADH Clinical – Hyperpituitarism, Hypopituitarism, local mass effects – radiographic abnormalities of the sella turcica, visual field abnormalities, elevated intracranial pressure, pituitary apoplexy Pituitary Gland Hyperpituitarism – pituitary adenoma Most common cause is adenoma arising in the anterior pituitary Classified based on the hormone produced Functional or nonfunctional Microadenoma < 1 cm Macroadenoma > 1 cm Usually soft, well-circumscribed 30% invasive adenomas – no capsule Cellular monomorphism and the absence of a significant reticulin network distinguish pituitary adenomas from non-neoplastic anterior pituitary parenchyma Atypical adenomas – p53 mutations, aggressive Pituitary Gland Prolactinomas Most frequent hyperfunctioning adenoma Amenorrhea, galactorrhea, loss of libido, infertility Tend to undergo dystrophic calcification Any mass in the suprasellar department may disturb the normal inhibitory influence of the hypothalamus (via dopamine secretion) on prolactin secretion resulting in hyperprolactinemia Pituitary Gland Somatothroph adenoma Second most common GH stimulates the hepatic secretion of IGF-1 (somatomedin C) Gigantism or acromegaly Failure to suppress GH production in response to a glucose challenge is one of the most sensitive tests for acromegaly Pituitary Gland Corticotroph adenoma Cushing disease Nelson syndrome Gonotroph adenoma Thyrotroph adenoma Nonfunctioning pituitary adenoma Pituitary carcinoma ( <1% of all pituitary tumors) Pituitary Gland Hypopituitarism Decreased secretion of pituitary hormones Hypofunction when > 75% of pituitary is lost or absent Causes – Tumors and other mass lesions, traumatic brain injury, subarachnoid hemorrhage, pituitary surgery or irradiation, pituitary apoplexy, ischemic necrosis and Sheehan syndrome, Rathke cleft cyst, empty sella syndrome, genetic defects, hypothalamic lesions, inflammatory or infections Pituitary Gland Posterior pituitary syndromes DI SIADH Hypothalamic suprasellar tumors Gliomas Craniopharygiomas Thyroid Gland Hyperthyroidism Hypothyroidism Cretinism Myxedema Thyroiditis Hashimoto thyroiditis Subacute (granulomatous) thyroiditis Subacute lymphocytic (painless) thyroiditis Graves disease Diffuse and multinodular goiters Diffuse nontoxic (simple) goiter Multinodular goiter Neoplasms of the thyroid Adenomas Carcinomas Pathogenesis Papillary carcinonoma Follicular carcinoma Anaplastic (undifferentiated) carcinoma Medullary carcinoma Congenital anomalies Thyroid Gland Hyperthyroidism Hypermetabolic state caused by elevated circulating levels of free T3 and T4 Thyrotoxicosis Most common forms: Diffuse hyperplasia associated with Graves disease ( 85%) Hyperfunctional multinodular goiter Hyperfunctional adenoma of the thyroid Thyroid GLand Clinical manifestations of hyperthyroidism Hypermetabolic state Overactivity of the sympathetic nervous system Warm, flushed skin heat intolerance Sweating Weight loss despite increased appetite Cardiac- tachycardia, palpitations, cardiomegaly, arrhythmias,CHF,cardiomyopathy Neuromuscular – tremor, hyperactivity, emotional lability, anxiety, inability to concentrate, insomnia, myopathy Ocular – wide staring gaze, lid lag Osteoporosis Thyroid storm Apathetic hyperthyroidism Thyroid Gland Hypothyroidism CausesPrimary – Thyroid dysgenesis, Thyroid hormone resistance syndrome, postablative, Hashomoto’s thyroiditis, Iodine deficiency, drugs, dyshormonogenetic goiter Penred syndrome (+hearing loss) Secondary – Pituitary failure, Hypothalamic failure Thyroid Gland Clinical manifestations: Cretinism – infancy or childhood, impaired development of the skeletal system and CNS, short stature and mental retardation Myxedema- older child or adult, slowing of physical and mental activity, fatigue, apathy, mental sluggishness, decreased sympathetic activity, non-pitting edema due to accumulation of matrix substances, decreased cardiac output Thyroid gland Thyroiditis Infectious- acute or chronic Hashimoto – autoimmune; antithyroglobulin, anti-thyroid peroxidase antibodies, Painless enlargement with hypothyroidism in a middle- aged woman, inflammatory infiltrate, germinal centers, Hurthle cells Subacute (granulomatous or DeQuervain) triggered by a viral infection, painful enlargement, transient Subacute lymphocytic (painless) – also post- partum, variant of Hashimoto Riedel – extensive fibrosis of thyroid and contiguous structures Thyroid Gland Graves disease Hyperthyroidism Infiltrative ophthalmopathy exothalmos Localized, infiltrative dermopathy pretibial myxedema Antibodies: Thyroid-stimulating immunoglobulin, thyroid growth-stimulating immunoglobulin, TSH-binding inhibitor immunoglobulin Diffuse hypertrophy and hyperplasia with tall, crowded follicular cells Thyroid Gland Diffuse nontoxic (simple) goiter- colloid goiter, iodine deficiency, clinically euthyroid, sporadic usually related to substances that interfere with thyroid hormone synthesis, mass effects from enlarging size Multinodular goiter- recurrent hyperplasia and involution from a long-standing simple goiter, mistaken for neoplasia, mass effects, occasionally toxic - hyperthyroidism Thyroid gland Neoplasms Adenoma Carcinoma Papillary Follicular Anaplastic Medullary Congenital anomaly – Thyroglossal duct or cyst Thyroid Gland Solitary nodules, in general, are likely to be neoplastic than are multiple nodules Nodules in younger patients are more likely to be neoplastic than are those in older patients Nodules in males are more likely to be neoplastic that are those in females A history of radiation treatment to the head and neck region is associated with an increased incidence of thyroid malignancy Functional nodules that take up radioactive iodine in imaging studies (hot nodules) are significantly more likely to be benign than malignant Thyroid Gland Adenomas Follicular, capsule, functioning autonomy, TSH receptor signaling pathway mutations in toxic ademonas Unilateral painless mass – usual presentation Thyroid Gland Carcinomas Papillary – 85% of cases, associated with prior radiation, Orphan Annie eyes nuclei, papillae, psammoma bodies Follicular Anaplastic Medullary - MEN syndromes, calcitonin Parathyroid Gland PTH Increases renal tubular reabsorption of calcium, thereby conserving free calcium Increases the conversion of vitamin D to its active dihydroxy from in the kidneys Increases urinary phosphate excretion, thereby lowering serum phosphate levels Augments gastrointestional calcium absorption Malignancy is the most common cause of clinically apparent hypercalcemia Hyperparathyroidism is the most common cause of asymptomatic hypercalcemia Parathyroid Glands Hyperparathyroidism Primary – adenoma ( 85-95%), hyperplasia, carcinoma Familial forms – MEN-1, MEN-2, Familial hypocalciuric hypercalcemia Cyclin D1gene inversions MEN1 mutations Clinical – “painful bones, renal stones, abdominal groans, psychic moans” Table 24-5 Causes of Hypercalcemia Secondary – renal failure is most common Hypoparathyroidism – surgically induced, autoimmune, Ad, FIH, congenital absence, tetany, Chvostek and Trousseau signs, mental status changes, intracranical calcifications, Prolonged QT, dental Pseudohypoparathyroidism The Endocrine Pancreas Diabetes mellitus Diagnosis Classification Glucose homeostasis Pathogenesis of type 1 DM Pathogenesis of type 2 DM Monogenic forms of diabetes Pathogenesis of late complications of DM Morphology of diabetes and its late complications Clinical features of DM Diabetes Mellitus Diagnosis A random glucose > 200mg/d l, with classical signs and symptoms Fasting glucose concentration > 126 mg/dl Abnormal oral glucose tolerance test ( glucose >200mg.dL 2 hours after a standard carbohydrate load “pre-diabetes” Diabetes Mellitus Classification – Table 24-6 Glucose homeostasis Glucose production in the liver Glucose uptake and utilization by peripheral tissues (primarily muscle) Actions of insulin and counter-regulatory hormones Most important stimulus for insulin synthesis and release is glucose itself Insulin is the most potent anabolic hormone, increase the rate of glucose transport into certain cells in the body – striated muscles including cardiac and adipose, other cells – uptake is insulin dependent Diabetes Mellitus Pathogenesis of Type I Autoimmune disease in which islet destruction is caused primarily by immune effector cells reacting against endogenous Beta-cell antigens HLA-DR3 or HLA-DR4 Viral infections Failure of self-tolerance in T cells Clinical manifestations begin after > 90% Beta cells are destroyed Diabetes Mellitus Pathogenesis of Type 2 Decreased response of the peripheral tissues to insulin Beta-cell dysfunction – manifested as inadequate insulin secretion in the face of insulin resistance and hyperglycemia Obesity Nonesterified fatty acids Adiokines Inflammation Peroxisome proliferator-activated receptor gamma Intrinsic predisposition to Beta-cell failure Pathogenesis of Complications of DM Macrovascular disease – accelerated atherosclerosis Microvascular disease – retinopathy, nephropathy, neuropathy Persistent hyperglycemia – Hemoglobin A1C Pormation of advanced glycation end products Activation of protein kinase C Intracellular hyperglycemia and disturbances in polyol pathways Morphology of DM and Complications Pancreas Reduction in number and size of islets Leucocytic inflitrates in the islets Amyloid deposition , reduction in islet cell mass ( type 2) Increase in number and size of islets ( IDM) Macrovascular disease Endothelial dysfunction, MI – most common cause of death, gangrene, hyaline arteriolosclerosis Diabetic microangiopathy Diffuse thickening of basement membranes, leaky capillaries Diabetic nephropathy Glomerular lesions – Thickening of GBM, increase in mesangial matrix, intercapillary glomerulosclerosis ( Kimmelstiel-Wilson) Renal vascular Pyelonephritis Diabetic ocular complications – chapter 29 Diabetic neuropathy – chapter 27 Clinical Features of DM Type 1 Polyuria, polydipsia, polyphagia Honeymoon period Catabolic state – glucose, fats, proteins DKA Table 24-7 Type 1 vs Type 2 DM Clinical Features Type 2 Hyperosmolar nonketotic coma Complications MI, Renal vascular insufficiency, strokes End-stage renal disease Visual impairment Distal symmetric polyneuropathy of lower extremities Autonomic neuropathy Increased susceptibility to infections: skin, TB, pneumonia, pyelonephritis The Endocrine Pancreas Pancreatic Endocrine Neoplasms Hyperinsulinism (insulinoma) – persistent hypoglycemia Zollinger-Ellison syndrome (gastrinomas) – peptic ulceration Other rare pancreatic endocrine neoplasms Alpha –cell tumors – increase glucagon Delta-cell tumors – somatostatinomas VIPoma Carcinoid Adrenal Glands Adrenal cortex Adrenocortical hyperfunction (hyperadrenalism) Hypercortisolism (Cushing syndrome) Primary hyperaldonsteronism Adrenogenital syndromes Adrenocortical insufficiency Primary acute adrenocortical insufficiency Waterhouse-Friderichsen syndrome Primary chronic adrenocortical insufficiency (Addison disease) Secondary adrenocortical insufficiency Adrenocortical neoplasms Other lesions of the adrenal Adrenal medulla Pheochromocytoma Adrenal Cortex Hypercortisolism ( Cushing syndrome) Clinical features – Table 24-9 Any condition that produces elevated glucocorticoid levels Exogenous – administration of gluocorticoids Endogenous – ACTH-dependent, ACTH –independent Table 24-8 Cushing disease – ACTH-producing pituitary microadenoma Secretion of ectopic ACTH Adrenal neoplasms Adrenal Cortex Primary Hyperaldosteronism Resultant suppression of renin-angiotensin system Decreased levels of renin Bilateral idipathic hyperaldosteronism Adrenocortical neoplasm Glucocorticoid-remediable hyperaldosteronism Hypertension – endothelial dysfunction Secondary caused by activation of renin-angiotensin system, increased renin Adrenal Cortex Adrenogenital syndrome Neoplasms Congenital adrenal hyperplasia 21-hydroxylase deficiency Salt-wasting Simple virilizing ( ambiguous genitalia) Nonlassic or late-onset adrenal virilism Adrenal Cortex Adrenocortical Insufficiency – Table 24-10 Acute adrenal cortical insufficiency Crisis precipitated by any form of stress Rapid withdrawal of steroids or failure to increase dose with acute stress Massive adrenal hemorrhage Weaknees, fatigue, hyperpigmentation (primary ), hyperkalemia, hypomatremia, volume depletion, hypotension Adrenal Medulla Paraganglion system Pheochromocytomas Rule of 10s – sort of Extra-adrenal Bilateral Malignant No hypertension Familial Multiple Endocrine Neoplasia Syndromes Multiple Endocrine Neoplasia, type 1 (Wermer syndrome) Parathyroid, pancreas, pituitary, also gastrinomas Multiple Endocrine Neoplasia, type 2 MEN-2A (Sipple syndorme) Pheochromocytoma, medullary carcinoma, parathyroid hyperplasia MEN -2B Neuromas, marfanoid habitus, similar to 2A withouf hyperparathyroidism Familial Medullary thyroid cancer -RET mutations, prophylactic thyroidectomy Pineal Gland Pinealomas Germinomas