* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CHAPTER 2
Bra–ket notation wikipedia , lookup
Abuse of notation wikipedia , lookup
Location arithmetic wikipedia , lookup
Large numbers wikipedia , lookup
Approximations of π wikipedia , lookup
History of mathematical notation wikipedia , lookup
Musical notation wikipedia , lookup
Big O notation wikipedia , lookup
Elementary mathematics wikipedia , lookup
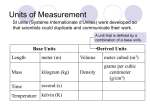
CHAPTER 2 Measurements and Calculations Scientific Method System Specific portion of matter that has been selected for study Scientific Method Logical approach to solve a problem Scientific Method Steps Observing and collecting data Use of senses Quantitative data – numerical Qualitative data - descriptive Generalization – statements Organizing – Graphs, tables, statistics Hypothesis – testable statement Law – statement that DESCRIBES facts Scientific Method Steps Theorizing Statements that EXPLAINS facts Can never be proven!! Testing Experimentation Units of Measurement Unit of Measurement A physical quantity of a defined size lb, in, ft, g, cm, km SI International System of Units (metric system) Adopted in 1960, originated in France SI SI base units – standard of measure Length – meter (m) Mass – gram (g) Time – second (s) Temperature – Kelvin (K) SI Prefixes Prefix Symbol Example Exponential Factor Factor Tera T Terameter 1012 1000000000000 Giga G Gigameter 109 1000000000 Mega M Megameter 106 1000000 Kilo K or k Kilometer 103 1000 Hecto H Hectometer 102 100 Deca D Decameter 101 10 ---- ---- meter 100 ---- Deci d Decimeter 10-1 0.1 Centi c Centimeter 10-2 0.01 Milli m Millimeter 10-3 0.001 Micro µ Micrometer 10-6 0.000001 Nano n Nanometer 10-9 0.000000001 Pico p Picometer 10-12 0.000000000001 Know the ones in BOLD above!!! SI Prefixes Number Line – MEMORIZE!! KHD Examples: d c m _ _ µ_ _ n Derived SI Units Derived Unit – obtained from combining base units Area Volume L * w * h ; m3 Speed L * w ; m2 Length/time ; m/s Density Mass/volume ; g/mL or g/cm3 Conversion Factors and Factor-Label Method Factor-Label Method – problem solving method using algebra Examples: Using Scientific Measurements Accuracy Precision Closeness of a measurement to the true or accepted value Agreement among the values Percent Error Accepted value – Experimental Value x 100% Accepted Value http://honolulu.hawaii.edu/distance/sci122/SciLab/L5/accprec.html Significant Figures Sig Figs – all certain digits plus one uncertain digit How many sig figs in a number? Table 2-5 page 47 Sig Figs Rules All non-zero numbers ARE significant Sandwich zeros ARE significant 306 = 3 SF Leading zeros ARE NOT significant 3.456 = 4 SF .000239 = 3 SF Trailing zeros: To the left – ARE NOT significant unless a special sign To the right – ARE significant 300 = 1 SF 300. = 3 SF 0.02300 = 4 SF Scientific Notation All digits in the number portion ARE significant 2.31 x 103 = 3 SF Significant Figures Using Sig Figs in Math Operations Multiply/Divide Answer must have number of sig figs as least precise number 2.3 (2 SF) x 5.67 (3 SF) = 13 (2 SF) 16.00 (4 SF) / 8.0 (2 SF) = 2.0 (2 SF) Add/Subtract Answer must have number of “columns” as least precise number 1.03 (hundredths) + 3 (ones) 4 Significant Figures Rounding off a number – Table 2-6 page 48 Rules – Decide where the number will be “cut” Look at number to the right: If it is a 5 or greater, increase the number by one If it is less than 5, leave number as is Significant Figures Examples: Scientific Notation Used to represent very big or very small numbers Generic form: M x 10N M must be greater than 1 and less than 10 If positive (+) N value = a “big” number If negative (–) N value = a “small” number Scientific Notation Example: 4.21 x 102 4.21 = number part in standard form (one digit to left of decimal point) 102 = tells where decimal is 2 = exponent Scientific Notation Converting TO Scientific Notation Count the number of spaces needed to get into PROPER form. This becomes the exponent. Moving the decimal point left means N is +. Moving the decimal point right means N is -. Examples: Scientific Notation Converting OUT OF scientific notation: Move the decimal the number of spaces indicated by the exponent (the number), the correct direction, also indicated by the exponent (the sign) Examples: Scientific Notation Calculator Type the “M” Hit the EE or EXP button Type the “N” Scientific Notation Math and scientific notation Add/Subtract Multiply Exponents MUST be the same!! Add M values and exponent stays the same Multiply M values and add exponents Divide Divide M values and subtract exponents Heat and Temperature Temperature Measure of the AVERAGE kinetic energy of the particles in a sample How hot or cold something is Heat SUM TOTAL of the kinetic energy of the particles in a sample More particles = more heat Heat and Temperature Thermometer Device used to measure temperature Hg or alcohol Liquid EXPANDS or CONTRACTS Temp scales °C – Celsius, 0°C, 100°C °F – Fahrenheit, 32°F, 212°F Heat and Temperature Kelvin Freezing point of water = 273 K Boiling point of water = 373 K K = °C + 273.15 °C = K – 273.15 Examples: Heat and Temperature Units of Heat Joule (J) – SI unit Calorie (cal) – older, not SI 1 cal = 4.184 J Problem Solving Analyze Plan Develop a plan to solve Compute Read problem carefully and analyze info Substitute data and conversion factors into plan and solve Evaluate Examine answers – is it reasonable? Does it make sense? Proportionality Variable Directly proportional Quantity that can change One goes up, other goes up; y=kx Graph – Inversely proportional One goes up, other goes down; y=k/x Graph –