* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biochem lectures
Nicotinamide adenine dinucleotide wikipedia , lookup
Citric acid cycle wikipedia , lookup
Signal transduction wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
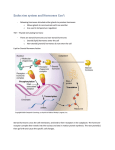
Introduction The use of enzymes in the diagnosis of disease is one of the important benefits derived from the intensive research in biochemistry since the 1940's Enzymes have provided the basis for the field of clinical chemistry It is, however, only within the recent past few decades that interest in diagnostic enzymology has multiplied Many methods currently on record in the literature are not in wide use, and there are still large areas of medical research in which the diagnostic potential of enzyme reactions has not been explored at all Early Enzyme Discoveries Some of the earliest studies were performed in 1835 by the Swedish chemist Jon Jakob Berzelius who termed their chemical action catalytic It was not until 1926, however, that the first enzyme was obtained in pure form, a feat accomplished by James B. Sumner of Cornell University Sumner was able to isolate and crystallize the enzyme urease from the jack bean. His work was to earn him the 1947 Nobel Prize John H. Northrop and Wendell M. Stanley of the Rockefeller Institute for Medical Research shared the 1947 Nobel Prize with Sumner. They discovered a complex procedure for isolating pepsin. Enzymes and Life Processes The living cell is the site of tremendous biochemical activity called metabolism This is the process of chemical and physical change which goes on continually in the living organism Build-up of new tissue, replacement of old tissue, conversion of food to energy, disposal of waste materials, reproduction - all the activities that we characterize as "life". The greatest majority of these biochemical reactions do not take place spontaneously The phenomenon of catalysis makes possible biochemical reactions necessary for all life processes Catalysis Catalysis is defined as the acceleration of a chemical reaction by some substance which itself undergoes no permanent chemical change The catalysts of biochemical reactions are enzymes and are responsible for bringing about almost all of the chemical reactions in living organisms Without enzymes, these reactions take place at a rate far too slow for the pace of metabolism Chemical Nature of Enzymes. Many enzymes require the presence of other compounds - cofactors - before their catalytic activity can be exerted. This entire active complex is referred to as the holoenzyme; i.e., apoenzyme (protein portion) plus the cofactor (coenzyme, prosthetic group or metal-ion-activator) is called the holoenzyme. Apoenzyme + Cofactor = Holoenzyme .1- A coenzyme - a non-protein organic substance which is dialyzable, thermostable and loosely attached to the protein part. .2- A prosthetic group - an organic substance which is dialyzable and thermostable which is firmly attached to the protein or apoenzyme portion. .3- A metal-ion-activator - these include K ,+Fe ,++Fe ,++Zn ,++Mg ,++Ca Specificity of Enzymes One of the properties of enzymes that makes them so important as diagnostic and research tools is the specificity they exhibit relative to the reactions they catalyze Other enzymes will be specific for a particular type of chemical bond or functional group In general, there are four distinct types of specificity: A- Absolute specificity - the enzyme will catalyze only one reaction. B- Group specificity - the enzyme will act only on molecules that have specific functional groups, such as amino, phosphate and methyl groups. C- Linkage specificity - the enzyme will act on a particular type of chemical bond regardless of the rest of the molecular structure. D- Stereochemical specificity - the enzyme will act on a particular steric or optical isomer. Enzymes can be classified by the kind of chemical reaction catalyzed 1- Addition or removal of water A-Hydrolases - these include esterases, carbohydrases, nucleases, deaminases, amidases, and proteases B-Hydrases such as fumarase, enolase, aconitase and carbonic anhydrase 2- Transfer of electrons A-Oxidases B-Dehydrogenases 3- Transfer of a radical A-Transglycosidases - of monosaccharides B-Transphosphorylases and phosphomutases - of a phosphate group C-Transaminases - of amino group B-Transmethylases - of a methyl group C-Transacetylases - of an acetyl group 4- Splitting or forming a C-C bond A-Desmolases 5- Changing geometry or structure of a molecule A-Isomerases 6- Joining two molecules through hydrolysis of pyrophosphate bond in ATP or other tri-phosphate A- Ligases Enzyme Kinetics: Energy Levels The enzyme is thought to reduce the "path" of the reaction. This shortened path would require less energy for each molecule of substrate converted to product. Given a total amount of available energy, more molecules of substrate would be converted when the enzyme is present (the shortened "path") than when it is absent. Hence, the reaction is said to go faster in a given period of time. Enzyme Kinetics: Basic Enzyme Reactions The basic enzymatic reaction can be represented as follows where E represents the enzyme catalyzing the reaction, S the substrate, the substance being changed, and P the product of the reaction. If this reaction is combined with the original reaction above equation the following results: Chemical Equilibrium The study of a large number of chemical reactions reveals that most do not go to true completion. This is likewise true of enzymatically-catalyzed reactions. This is due to the reversibility of most reactions. In general: where K 1+is the forward reaction rate constant and K 1-is the rate constant for the reverse reaction. Combining the two reactions gives: Applying this general relationship to enzymatic reactions allows the equation: Equilbrium, a steady state condition, is reached when the forward reaction rates equal the backward rates. This is the basic equation upon which most enzyme activity studies are based. Sugars Prefer To Be Cyclic Carbohydrates Are Chiral Molecules Typically but not always • L – amino acids D • D - sugars Hence, these molecules have a measurable optical rotation, which depends upon both the monomer residues and their conformation L Glyceraldehyde Fisher Formulas Next to last carbon determines D or L New carbon is added as C1 Hormones A hormone Is a chemical messenger that carries a signal from one cell( or group of cells) to another via the blood. hormone : "A chemical secreted by cells in one part of the body that is transported in the bloodstream to other parts of the body, where it affects particular target cells ". -example: hypothalamus, pituitary All multicellular organisms produce hormones Endocrine hormone molecules are secreted (released) directly into the bloodstream ,while exocrine hormones (or ectohormones) are secreted directly into a duct, and from the duct they either flow into the bloodstream or they flow from cell to cell by diffusion in a process known as paracrine signalling. Hierarchical nature of hormonal control Hormonal regulation of some physiological activities involves a hierarchy of cell types acting on each other either to stimulate or to modulate the release and action of a particular hormone. The secretion of hormones from successive levels of endocrine cells is stimulated by chemical signals originating from cells higher up the hierarchical system. The master coordinator of hormonal activity in mammals is the hypothalamus , which acts on input that it receives from the central nervous system Other hormone secretion occurs in response to local conditions, such as the rate of secretion of parathyroid hormone by the parathyroid cells in response to fluctuations of ionized calcium levels in extracellular fluid. Function of hormones HOMEOSTASIS Reproduction Growth and development Maintenance of internal environment Production, utilization and storage of energy Chemical nature of hormones Can be divided into 3 Groups: Amino acid derivatives Peptide hormones Lipid derivatives Amino acid derivatives Derivatives of tyrosine Catecholamines (epinephrine,dopamine) Thyroid hormones (dipeptides) Tryptophan derivative Melatonin Peptide hormones Glycoproteins from anterior pituitary thyroid-stimulating hormone (TSH) luteinizing hormone (LH) follicle-stimulating hormone (FSH) Peptides and small proteins Digestive tract hormones Pituitary hormones Pancreatic hormones classes of lipid derived hormones Steroid hormones: derived from cholesterol 2 groups with the intact steroid ring (adrenal and gonadal steroids) with the steroid ring cleaved (metabolites of vit D) Eicosanoids: derived from arachidonic acid Hormone receptors Molecules within or on the surface of target cells that bind hormones with high affinity and specificity and thereby initiate and mediate biological responses Hormones will only produce the response in cells that express the receptors for this particular hormone (target cells) ONLY target cells respond to hormone Cells that do not have receptors for the hormone “ignore” the hormone Steroid hormones The steroid hormones are all derived from cholesterol. Moreover, with the exception of vitamin D ,they all contain the same cyclopentanophenanthrene ring and atomic numbering system as cholesterol. The important mammalian steroid hormones are shown below along with the structure of the precursor, pregneolone. Retinoic acid and vitamin D are not derived from pregnenolone, but from vitamin A and cholesterol respectively . Vitamins History Purified diets of carbohydrate, protein, fat, minerals and water were not capable of normal growth “Accessory growth factors” Casimir Funk, a Polish biochemist, isolated an antiberberi substance from rice polishings Named it vitamine, an amine, vital for life Vitamins Essential organic compounds required in very small amounts (micronutrients) involved in fundamental functions of the body Unrelated chemically Vitamins Not metabolic fuels (like glucose or fatty acids) or structural nutrients (like amino acids) Regulators (catalysts) of reactions, some of which are involved in energy Metabolism Organic molecules in food Required in small amounts Classified based on solubility Fat soluble Water soluble Classification of vitamins Fat-soluble Vitamins Water-soluble Vitamins Vitamins All vitamins are metabolically essential but not all required in the diet Most mammals can synthesize vitamin C; not humans and primates No mammal can synthesize B vitamins but rumen bacteria do The Basics of Water-Soluble Vitamins Dissolve in water B vitamins & vitamin C Absorbed mostly in small intestine & stomach Bioavailability Nutritional status, other nutrients & substances in food, medications, age, illness Circulated to liver in blood Not stored in large quantities The Basics of Water-Soluble Vitamins Naming the Vitamins First named vitamin A or B B-complex vitamins Given common names also Thiamin Riboflavin Niacin Chemical names Ascorbic acid Thiamin (Vitamin B1) Contains thiol & amine group (-SH) and (NH3) Thiamin pyrophosphate (TPP) or thiamin diphosphate Thiamin triphosphate Functions of Thiamin ATP production Synthesis of DNA & RNA Noncoenzyme roles Coenzyme Functions of Riboflavin (B2) Energy metabolism Redox reactions Formation of ATP, water, carbon dioxide β-oxidation Converts vitamin A & folate to active forms, tryptophan to niacin Forms vitamin B6 & K Riboflavin Deficiency Ariboflavinosis Weakness, cheilosis, stomatitis, glossitis, anemia, confusion Alcoholics Diseases that interfere w/ riboflavinutilization Regulation of Vitamin B12 in the Body Must be cleaved before absorption Bound to R protein & intrinsic factor Once absorbed, binds to transcobalamin Circulates to liver via blood Stored in liver Functions of Vitamin B12 Coenzyme that catalyzes: Production of succinyl CoA Uses amino acids & fatty acids for ATP production Conversion of homocysteine to methionine Allows use of folate Regulation of Vitamin C in the Body Absorption in small intestine via active transport Uses glucose transport protein High intakes Absorbed by simple diffusion in stomach & small intestine Circulates to liver via blood Excess excreted in urine Functions of Vitamin C Antioxidant Accepts & donates electrons Involved in a variety of redox reactions