* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Piriformis pyomyositis: a report of three cases
Survey
Document related concepts
Transcript
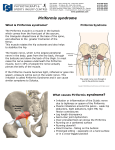
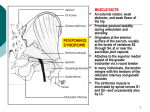
Journal of Orthopaedic Surgery 2008;16(3):389-91 Piriformis pyomyositis: a report of three cases CH Wong, SH Choi, KY Wong Department of Orthopaedics and Traumatology, Princess Margaret Hospital, Hong Kong ABSTRACT Pyomyositis is a subacute infection of skeletal muscles. It can be life-threatening if diagnosis and treatment are delayed. Only 5 cases of isolated piriformis pyomyositis have been reported. We report signs and symptoms of piriformis pyomyositis in 3 women who were treated mainly with antibiotics. Computed tomography is useful in making the diagnosis. Early diagnosis and treatment may avoid surgical treatment and reduce mortality. Key words: infection; pyomyositis; sciatica INTRODUCTION Pyomyositis is a subacute infection of skeletal muscles, most commonly involving the quadriceps, followed by the gluteal muscles and iliopsoas,1,2 with a mortality rate of up to 3.6% of cases.3 It can be primary or secondary and more commonly occurs in patients in tropical and subtropical areas aged 2 to 5 years and 35 to 40 years because of kwashiorkor and marasmus.4,5 It is also known as tropical myositis, myositis tropicans, purulenta tropica, and tropical pyomyositis. The incidence of pyomyositis has been rising in temperate areas, predominantly in men; half of the cases in North America have been in intravenous drug abusers or immunosuppressed patients with rheumatoid diseases, malignancies, diabetes mellitus, chronic liver diseases, or acquired immune deficiency syndrome.3,6–11 Only 5 cases of isolated piriformis pyomyositis have been reported.6,12–15 We report signs and symptoms of piriformis pyomyositis in 3 women who were treated mainly with antibiotics. One of whom had drainage. CASE REPORTS Case 1 In July 2006, a 45-year old woman presented with lower back pain radiating to the left leg. She was afebrile and initially diagnosed with sciatica and observed for 3 days. The pain progressively increased and she developed a high fever (39ºC) and could not walk or sleep. Her left buttock was tender and the pain was increased by internal rotation of the left hip, limiting the straight leg raise. There was no neurological deficit of the leg. Inflammatory markers were elevated (white cell count [WCC], 14.5 x109/l; erythrocyte sedimentation rate [ESR], 49 mm/h; Creactive protein [CRP], 241 mg/l). Radiographs of the lumbrosacral spine were normal. Computed tomography (CT) showed abscess formation in the left pelvis, with extension to the iliacus and piriformis. She was empirically treated with intravenous cefazolin 1 g every 8 hours for one week. Blood culture grew Address correspondence and reprint requests to: Dr Chong-hing Wong, Resident Medical Officer, Department of Orthopaedics and Traumatology, Princess Margaret Hospital, Lai Chi Kok, Hong Kong. E-mail: nelsonwchi@yahoo.com.hk Journal of Orthopaedic Surgery 390 CH Wong et al. Staphylococcus aureus and antibiotics were changed to intravenous cloxacillin 1 g every 8 hours for 5 days and oral cloxacillin 1 g 4 times daily for 6 weeks. The pain gradually subsided and inflammatory markers returned to normal as the abscess resolved. (a) Case 2 In January 2007, a 58-year-old woman presented with tachycardia and high fever after a 2-week history of progressive lower back and buttock pain and a oneweek history of fever, chills, and rigours. Inflammatory markers were elevated (WCC, 22 x109/l; ESR, 71 mm/h; CRP, 302 mg/l). Radiographs of the lumbrosacral spine were unremarkable. She was empirically treated with intravenous augmentin 1.2 g every 8 hours, but remained febrile and hypotensive, requiring inotrope support. Blood culture was negative. Antibiotics were changed to intravenous meropenem 500 mg every 8 hours and flagyl 500 mg every 8 hours because of the persistent fever. A CT scan showed an abscess in the right piriformis and a small pre-forming abscess in the left piriformis. Following CT-guided drainage of the right piriformis abscess, the fever and pain subsided. The aspirate yielded negative culture but showed numerous neutrophils, compatible with an acute inflammatory exudate. The abscesses resolved after 2 weeks of intravenous augmentin 1.2 g every 8 hours and 3 weeks of oral augmentin 375 mg 3 times daily. (b) Figure Transverse and coronal computed tomographic images showing the piriformis abscess (arrows). Case 3 In May 2007, a 71-year-old woman presented with a one-day history of fever and pain in the left buttock and lower abdomen. Inflammatory markers were elevated (WCC, 15.1 x109/l; ESR, 96 mm/h; CRP, 186 mg/l). Radiographs of the lumbrosacral spine showed degenerative changes only. Ultrasonography of the abdomen showed bilateral hydronephrosis. A Foley catheter was inserted but the right hydronephrosis persisted. Cystoscopy was performed and bilateral ureteric catheters were inserted. She was empirically treated with intravenous cefuroxime 750 mg every 8 hours. Urine and blood culture grew S aureus and the antibiotics were changed to intravenous cloxacillin 1 g every 8 hours. A CT scan showed myositis along the left piriformis (Fig.). The pain and fever subsided and the myositis resolved after 2 weeks of intravenous cloxacillin 1 g every 8 hours and 4 weeks of oral cloxacillin 1 g 4 times daily. A retrograde pyelogram showed that the right hydronephrosis was likely due to right pelviureteric junction obstruction and left duplex kidney—the predisposing factor for the urinary tract infection. DISCUSSION Muscle is intrinsically resistant to infection unless traumatised. Animal studies have shown that intravenous injection of sublethal doses of S aureus failed to initiate infection unless muscles were traumatised by electric shock, pincing, or ischaemia.16 In a case of piriformis pyomyositis involving a swimmer, repeated backstrokes were considered the cause of the damage and subsequent infection of the piriformis.15 Other predisposing factors include vitamin C deficiency, beriberi, and parasite, virus or spirochaete infection. Human immunodeficiency virus (HIV) myositis and the mitochondrial toxicity of antiretroviral medications also increase the risk of infection.3,17 The most common causative organism is S aureus, accounting for 95% and 70% of the cases in tropical and temperate areas, respectively. Other organisms include streptococcal species, Gram-negative bacilli, and anaerobes. Gram-negative organisms are more common among immunosuppressed patients, who Vol. 16 No. 3, December 2008 usually present with a normal WCC and are more susceptible to multifocal involvement, with a higher mortality rate.3 The progress of infection and presentation can be divided into 3 stages.5 Stage 1 is the invasive stage, with minimal inflammation and no pus collection. The muscle is wooden or ‘hard-rubber’ stiff on palpation. Stage 2 is the suppurative stage, occurring 10 to 21 days after symptom onset, with most patients presenting at this stage. In stage 3, patients present with systemic upset, with high fever and septicaemia. A diagnosis of pyomyositis is made when the patient has any 3 of the following: (1) a recent visit to the tropics or subtropics, (2) eosinophilia of >10% with muscle tenderness, (3) tenderness and oedema in the susceptible skeletal muscle, and (4) needle aspiration of muscle yielding pus and exclusion of parasite infection.5 These criteria are less valid in developed countries because of acquired immune deficiency syndrome and other immunosuppressive diseases. Besides, 80% of cases in tropical areas have eosinophilia, probably secondary to parasite infection, but this is not the situation in temperate areas.5 Swelling of the piriformis secondary to injury or inflammation may compress the nerve and cause sciatica. The endopelvis fascia overlies the psoas, iliacus, piriformis, and obturator muscles and provides a route for infection from the spine or pelvis to the piriformis.12 Two cases of piriformis Piriformis pyomyositis 391 pyomyositis were reported in patients who had had obstetrical intervention. Only 5 cases of isolated piriformis pyomyositis have been reported6,12–15; all presented with sciatica and were likely to be positive to Pace’s test (pain and lower weakness with resisted hip external rotation and abduction) and Freiberg’s sign (pain with passive internal rotation of the hip). A tender, sausageshaped swelling, palpable on pre-rectal examination was reported in a case of piriformis syndrome.16 Inflammatory markers are usually elevated but muscle enzyme levels are generally normal. Only 13% and 19% of HIV-infected and HIV-negative patients, respectively, had elevated muscle enzyme levels.3 Contrast CT and magnetic resonance imaging are useful for diagnosis.9 Antibiotics and drainage are the mainstay of treatment, which may be combined with decompression of the sciatic nerve by tenotomy of the piriformis tendon at its musculo-tendinous junction near the greater trochanter.14 Three to 4 weeks of antibiotic use (with initial intravenous treatment) has been suggested,3 with immune status and the number of abscesses taken into account.3,18 In our series, the mean time to diagnosis was 5 days. A high index of suspicion is the key to making the diagnosis. The disease can be life-threatening if treatment is delayed. Early diagnosis and treatment may avoid surgical treatment and reduce mortality. REFERENCES 1. 2. 3. 4. Sokolowski MJ, Koh JL. Pyomyositis of the shoulder girdle. Orthopedics 2006;29:1030–2. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Primary pyomyositis. J Bone Joint Surg Am 2002;84:2277–86. Crum NF. Bacterial pyomyositis in the United States. Am J Med 2004;117:420–8. Rasmussen AE, Hansen TT, Tilma KA, Ehlers RW. Pyomyositis in children—a diagnostic challenge [in Danish]! Ugeskr Laeger 2007;169:2125–6. 5. Chiedozi LC. Pyomyositis. Review of 205 cases in 112 patients. Am J Surg 1979;137:255–9. 6. Colmegna I, Justiniano M, Espinoza LR, Gimenez CR. Piriformis pyomyositis with sciatica: an unrecognized complication of “unsafe” abortions. J Clin Rheumatol 2007;13:87–8. 7. Kaushik RM, Kaushik R, Sharma A, Mahajan SK. Tropical pyomyositis in a case of rheumatoid arthritis. Trans R Soc Trop Med Hyg 2006;100:895–8. 8. Villamil-Cajoto I, Maceiras-Pan F, Villacian-Vicedo MJ. Pyomyositis: report of seventeen cases [in Spanish]. Rev Med Chil 2006;134:31–8. 9. Drosos G. Pyomyositis. A literature review. Acta Orthop Belg 2005;71:9–16. 10. Hall RL, Callaghan JJ, Moloney E, Martinez S, Harrelson JM. Pyomyositis in a temperate climate. Presentation, diagnosis, and treatment. J Bone Joint Surg Am 1990;72:1240–4. 11. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis 1992;15:668–77. 12. Chong KW, Tay BK. Piriformis pyomyositis: a rare cause of sciatica. Singapore Med J 2004;45:229–31. 13. Kinahan AM, Douglas MJ. Piriformis pyomyositis mimicking epidural abscess in a parturient. Can J Anaesth 1995;42:240–5. 14. Chen WS. Sciatica due to piriformis pyomyositis. Report of a case. J Bone Joint Surg Am 1992;74:1546–8. 15. Chusid MJ, Hill WC, Bevan JA, Sty JR. Proteus pyomyositis of the piriformis muscle in a swimmer. Clin Infect Dis 1998;26:194–5. 16. Jankiewicz JJ, Hennrikus WL, Houkom JA. The appearance of the piriformis muscle syndrome in computed tomography and magnetic resonance imaging. A case report and review of the literature. Clin Orthop Relat Res 1991;262:205–9. 17. Shepherd JJ. Tropical myositis: is it an entity and what is its cause? Lancet 1983;2:1240–2. 18. Crum-Cianflone NF. Infection and musculoskeletal conditions: infectious myositis. Best Pract Res Clin Rheumatol 2006;20:1083–97.