* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atomic Structure ppt File
Survey
Document related concepts
Transcript
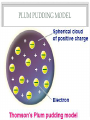
BELL WORK • What were the two most popular experiments that helped develop the atomic model and theory? • What is an isotope? • What makes isotopes different? STRUCTURE OF THE ATOM MR. SCHLAMB THE NUCLEUS • https://www.youtube.com/watch?v=FSyAehMdpyI CATHODE RAY TUBES • These cathode ray tubes led to the first discovery of a subatomic particle the electron • It was found that when the ray was exposed to a negative electric field the ray would bend away from the field • This led to the conclusion that negatively charged particles composed the ray and these became named electrons INFERENCES BASED ON ELECTRONS 1. Since atoms are electrically neutral they must contain a positive charge to balance the negative electrons 2. Because electrons have so much less mass than atoms, atoms must contain other particles that account for most of their mass • With this knowledge the first model of the atoms was purposed PLUM PUDDING MODEL RUTHERFORD GOLD FOIL EXPERIMENT • This led to the discovery of the atomic nucleus • https://www.youtube.com/watch?v=_uEFKG122dA • Rutherford concluded that the center of an atom which he called a nucleus contained positive particles but was confused on where the electrons were NIELS BOHR • One of Rutherford's associates purposed a new model for the atom: Bohr’s model OIL DROP EXPERIMENT • https://www.youtube.com/watch?v=UFiPWv03f6g NUCLEUS • Usually like charges repel but in the nucleus 83 protons can exist and the nucleus still be stable • The force that holds an atomic nucleus together is referred to as nuclear forces ISOTOPES • Isotopes have the same number of protons but a different number of neutrons • This difference in neutrons changes the mass number