* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Structure of the atom
Survey
Document related concepts
Transcript
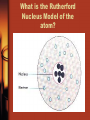
Structure of the atom What makes up the atom? • Nucleus – Protons (positively charged) – Neutrons (no overall charge) • Orbitals – Electrons (negatively charged) – Outermost orbital is called the valance shell. – Electrons in valance shell are called valance electrons. – Fill starting with the innermost orbital What is the Planetary Model of the atom? What is the Rutherford Nucleus Model of the atom? What is the Electron Cloud Model of the atom? How to read the periodic table? Name of Element Potassium K 19 Atomic Number Atomic Symbol 39.0983 Atomic Mass/ Weight (Mass Number) How to calculate what makes up an atom? Answers: Atomic Mass = 39 Potassium Atomic Number = 19 Protons = 19 19 K Electrons = 19 Neutrons = 20 39.0983 Atomic Number Atomic Mass/ Weight (Round to the nearest whole number when using in calculations) How to calculate Protons, Neutrons, and Electrons: # Protons = Atomic Number # Electrons = # Protons in a neutral atom # Neutrons = Atomic Mass (rounded) – Atomic Number You try Potassium……. What is element notation? X represents the atom's elemental symbol. A represents the atomic mass Z represents the atomic number Set up the element notation for Potassium (K) Let’s do an example Element = Carbon Atomic mass = 12 Atomic number = 6 Protons = ? Neutrons = ? Electrons = ? Element = Carbon Atomic mass = 12 Atomic number = 6 Protons = 6 Neutrons = 6 Electrons = 6