* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 15. Lateral Plate Mesoderm and Endoderm
Survey
Document related concepts
Transcript
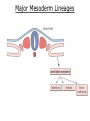
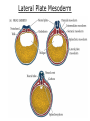
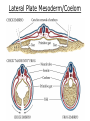
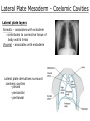
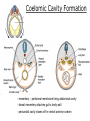
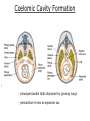
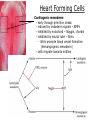
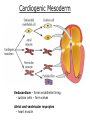
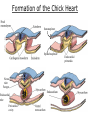
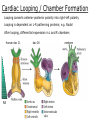
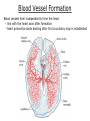
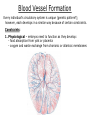
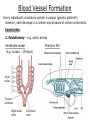
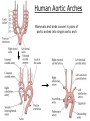
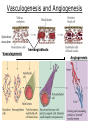
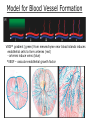
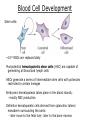
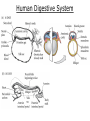
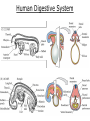
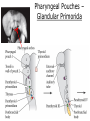
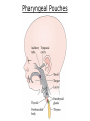
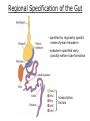
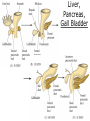
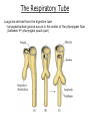
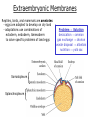
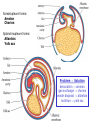
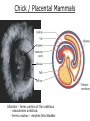
Developmental Biology Lateral Plate Mesoderm and Endoderm July 30, 2008 Major Mesoderm Lineages Lateral Plate Mesoderm Lateral Plate Mesoderm/Coelom Lateral Plate Mesoderm Coelomic Cavities Lateral plate layers Somatic – associates with ectoderm contributes to connective tissue of body wall & limbs Visceral – associates with endoderm Lateral plate derivatives surround coelomic cavities pleural pericardial peritoneal Coelomic Cavity Formation mesentery – peritoneal membrane lining abdominal cavity dorsal mesentery attaches gut to body wall pericardial cavity closes off in ventral anterior coelom Coelomic Cavity Formation pleuropericardial folds displaced by growing lungs pericardium forms as separate sac Lateral Plate Mesoderm and Endoderm Overview Coelomic cavity formation Cardiovascular development Heart Blood vessel Vasculogenesis Angiogenesis Blood cells Endoderm Digestive system Pharyngeal pouches Liver, lung, etc. Extraembryonic membranes Heart Forming Cells Cardiogenic mesoderm early through primitive streak induced by endoderm signals – BMPs inhibited by notochord – Noggin, chordin inhibited by neural tube – Wnts Wnts promote blood vessel formation (hemangiogenic mesoderm) cells migrate towards midline Cardiogenic Mesoderm Endocardium – forms endothelial lining; cushion cells – form valves Atrial and ventricular myocytes – heart muscle Formation of the Chick Heart Somatopleure Splanchnopleure Neural tube Foregut Endocardium Endocardial tube Pericardial cavity Endocardial primordia Cardiac Looping / Chamber Formation Looping converts anteriorposterior polarity into rightleft polarity Looping is dependent on LR patterning proteins; e.g. Nodal After looping, differential expression in L and R chambers Human day 21 RA day 28 newborn Blood Vessel Formation Blood vessels form independently from the heart link with the heart soon after formation heart primordia starts beating after first circulatory loop is established Blood Vessel Formation Every individual’s circulatory system is unique (genetic pattern?); however, each develops in a similar way because of certain constraints. Constraints: 1. Physiological – embryos need to function as they develop: food absorption from yolk or placenta oxygen and waste exchange from chorionic or allantoic membranes Blood Vessel Formation Every individual’s circulatory system is unique (genetic pattern?); however, each develops in a similar way because of certain constraints. Constraints: 2. Evolutionary – e.g. aortic arches Vertebrate model (e.g. human – 29 days) Primitive fish Human Aortic Arches Mammals and birds convert 6 pairs of aortic arches into single aortic arch Blood Vessel Formation Every individual’s circulatory system is unique (genetic pattern?); however, each develops in a similar way because of certain constraints. Constraints: 1. Physiological – embryos need to function as they develop food absorption from yolk or placenta oxygen and waste exchange from chorionic or allantoic membranes 2. Evolutionary – e.g. aortic arches 3. Physical – laws of fluid movement and diffusion constrain the size and number of vessels most effective fluid transport in large tubes however, diffusion of nutrients and gases can take place only through small tube and at slow flow Blood Vessel Formation Vasculogenesis – first process: capillary network formed from lateral plate mesoderm Angiogenesis – second process: primary capillary networks are remodeled; veins and arteries made Vasculogenesis and Angiogenesis Splanchnic mesoderm Vasculogenesis hemangioblasts Angiogenesis Model for Blood Vessel Formation VEGF* gradient (green) from mesenchyme near blood islands induces endothelial cells to form arteries (red) arteries induce veins (blue) *VEGF – vascular endothelial growth factor Arterial and Venous Differentiation Angiogenesis How to make sure veins attach just to arteries, and vice versa? Artery precursor endothelial cells contain ephrinB Vein precursors contain ephrin receptor Ephb4 tyrosine kinase Blood Cell Development Stem cells: ~10 11 RBCs are replaced daily Pluripotential hematopoietic stem cells (HSC) are capable of generating all blood and lymph cells HSCs generate a series of intermediate stem cells with potencies restricted to certain lineages Embryonic hematopoiesis takes place in the blood islands; mostly RBC production Definitive hematopoietic cells derived from splanchnic lateral mesoderm surrounding the aorta later move to the fetal liver; later to the bone marrow Blood Stem Cells Lateral Plate Mesoderm and Endoderm Overview Mesoderm lineages Cardiovascular development Heart Blood vessel Vasculogenesis Angiogenesis Blood cells Endoderm Digestive system Pharyngeal pouches Liver, lung, etc. Extraembryonic membranes Endoderm Endoderm has two functions: 1. Instructions for the formation of notochord, heart, blood vessels, mesoderm 2. Construction of linings of the digestive tube (including liver, gall bladder, and pancreas), and respiratory tube both the respiratory and digestive tubes are products of the primitive gut Human Digestive System Human Digestive System Pharyngeal Pouches – Glandular Primorida Pharyngeal Pouches Regional Specification of the Gut specified by regionally specific mesenchymal mesoderm endoderm specified early; possibly before tube formation transcription factors Liver, Pancreas, Gall Bladder The Respiratory Tube Lungs are derived from the digestive tube laryngeotracheal groove occurs in the center of the pharyngeal floor (between 4 th pharyngeal pouch pair) Lung Maturation Surfactant Lungs fully differentiate late in development; alveolar (air sac) cells secrete a surfactant; allows cells to slide against one another secreted late in gestation; ~ wk 34 (human) Lung Maturation Surfactant Lungs fully differentiate late in development; alveolar (air sac) cells secrete a surfactant; allows cells to slide against one another secreted late in gestation; ~ wk 34 (human) birth may be triggered by maternal immune response to surfactant Extraembryonic Membranes Reptiles, birds, and mammals are amniotes eggs are adapted to develop on dry land adaptations use combinations of ectoderm, endoderm, &mesoderm to solve specific problems of land eggs Somatopleure Splanchnopleure Problem → Solution desiccation → amnion gas exchange → chorion waste disposal → allantois nutrition → yolk sac Somatopleure forms: Amnion Chorion Splanchnopleure forms: Allantois Yolk sac Problem → Solution desiccation → amnion gas exchange → chorion waste disposal → allantois nutrition → yolk sac Chick / Placental Mammals Allantois – forms portion of the umbilicus vascularizes umbilicus forms urachus – empties fetal bladder