Chapter 22: Phenols. Alcohols contain an OH group bonded to an
... 22.3: Physical Properties (please read). Like other alcohols the OH group of phenols can participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
... 22.3: Physical Properties (please read). Like other alcohols the OH group of phenols can participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
120 Chapter 24: Phenols. Alcohols contain an OH group bonded to
... 24.3: Physical Properties (please read). Like other alcohols the OH group of phenols cab participate in hydrogen bonding with other phenol molecules and to water. 24.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
... 24.3: Physical Properties (please read). Like other alcohols the OH group of phenols cab participate in hydrogen bonding with other phenol molecules and to water. 24.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
ppt
... 24.3: Physical Properties (please read). Like other alcohols the OH group of phenols cab participate in hydrogen bonding with other phenol molecules and to water. 24.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
... 24.3: Physical Properties (please read). Like other alcohols the OH group of phenols cab participate in hydrogen bonding with other phenol molecules and to water. 24.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
Get PDF - Wiley Online Library
... of the TMS-enol ether of 2 (TMSOTf, Et3N), cleanly provided the C10 tertiary alcohol (18, 99 %). This was then utilized to set the adjacent C11 alcohol stereocenter through hydroxydirected reduction with Me4NBH(OAc)3 (87 %, > 19:1 d.r.).[22] TES protection proved necessary to facilitate the subseque ...
... of the TMS-enol ether of 2 (TMSOTf, Et3N), cleanly provided the C10 tertiary alcohol (18, 99 %). This was then utilized to set the adjacent C11 alcohol stereocenter through hydroxydirected reduction with Me4NBH(OAc)3 (87 %, > 19:1 d.r.).[22] TES protection proved necessary to facilitate the subseque ...
$doc.title
... • ReacHon of alcohols with Ag2O directly with alkyl halide forms ether in one step • Glucose reacts with excess iodomethane in the presence of Ag2O to generate a pentaether in 85% yield ...
... • ReacHon of alcohols with Ag2O directly with alkyl halide forms ether in one step • Glucose reacts with excess iodomethane in the presence of Ag2O to generate a pentaether in 85% yield ...
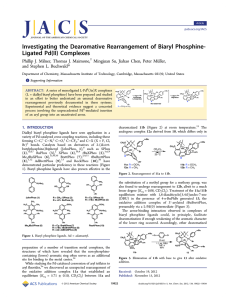
Investigating the Dearomative Rearrangement of Biaryl Phosphine- Ligated Pd(II) Complexes
... to occur. Taken together, these reports suggest that the lower ring of biaryl phosphine ligands may not be innocent in the reactivity and decomposition pathways of catalytic intermediates. Reactions wherein a transition metal-bound arene undergoes nucleophilic attack are well-established,18 as are a ...
... to occur. Taken together, these reports suggest that the lower ring of biaryl phosphine ligands may not be innocent in the reactivity and decomposition pathways of catalytic intermediates. Reactions wherein a transition metal-bound arene undergoes nucleophilic attack are well-established,18 as are a ...
Chem 2423-Test 2 - HCC Learning Web
... 22. pinene, C10H18 23. diazepam (Valium), C16H13N2OCl Draw structures corresponding to each name below. Either condensed or line structures may be used. 24. 3,6-dimethyl-1,4-cyclohexadiene ...
... 22. pinene, C10H18 23. diazepam (Valium), C16H13N2OCl Draw structures corresponding to each name below. Either condensed or line structures may be used. 24. 3,6-dimethyl-1,4-cyclohexadiene ...
Metal-catalysed approaches to amide bond formation
... Dimeric species 5 in which both zirconium centres are hexacoordinate is formed in the presence of amines. Coordination of an ester to one of the zirconium centres results in formation of either 6 or 7 by breaking of one bridging Zr–O bonds. Nucleophilic attack of the amine onto the ester then procee ...
... Dimeric species 5 in which both zirconium centres are hexacoordinate is formed in the presence of amines. Coordination of an ester to one of the zirconium centres results in formation of either 6 or 7 by breaking of one bridging Zr–O bonds. Nucleophilic attack of the amine onto the ester then procee ...
New Phenylglycine-Derived Primary Amine Organocatalysts for the
... anticipated by us that introducing sterically more demanding side chains through cyclohexylglycine or tert-leucine (amino alcohols 16–17) would enhance the enantioselectivity even further, a hypothesis obviously invalidated by experiment. Clearly, the presence of an aromatic moiety is crucial. Among ...
... anticipated by us that introducing sterically more demanding side chains through cyclohexylglycine or tert-leucine (amino alcohols 16–17) would enhance the enantioselectivity even further, a hypothesis obviously invalidated by experiment. Clearly, the presence of an aromatic moiety is crucial. Among ...
Chapter 19
... Amine salts are also used to catalyze a variety of organic reactions that feature two components that are soluble in different liquid phases (e.g. organic and aqueous) ...
... Amine salts are also used to catalyze a variety of organic reactions that feature two components that are soluble in different liquid phases (e.g. organic and aqueous) ...
Amines and amides
... This reaction involves breaking the C-N bond at the carbonyl carbon. a. acid hydrolysis: forms “ammonium” ion and carboxylic acid b. alkaline hydrolysis: forms carboxylate ion and amine Condensation Polymers As we have seen –COOH (and COCl) react with amines to form 2ry amides A Condensation reactio ...
... This reaction involves breaking the C-N bond at the carbonyl carbon. a. acid hydrolysis: forms “ammonium” ion and carboxylic acid b. alkaline hydrolysis: forms carboxylate ion and amine Condensation Polymers As we have seen –COOH (and COCl) react with amines to form 2ry amides A Condensation reactio ...
Direct organocatalytic enantioselective a-aminomethylation
... Trost and co-workers12 developed di-nuclear zinc organometallic complexes as catalyst for the direct catalytic enantioselective Mannich-type reactions between hydroxyarylketones and preformed imines. In addition, Jørgensen and co-workers have developed elegant direct asymmetric Mannich reactions inv ...
... Trost and co-workers12 developed di-nuclear zinc organometallic complexes as catalyst for the direct catalytic enantioselective Mannich-type reactions between hydroxyarylketones and preformed imines. In addition, Jørgensen and co-workers have developed elegant direct asymmetric Mannich reactions inv ...
Anionic rearrangement of 2-benzyloxypyridine derivatives and a synthetic approach to aldingenin B
... TABLE OF CONTENTS List of Tables .......................................................................................................... vii List of Figures ........................................................................................................ viii Abstract .................... ...
... TABLE OF CONTENTS List of Tables .......................................................................................................... vii List of Figures ........................................................................................................ viii Abstract .................... ...
CHM238-01 EXAM 2 October 14, 2002 103
... (c) Which reagent(s) would you use if you wanted to substitute the alcohol with bromide in the same position and with inversion of configuration, without any rearrangement. (d) This type of rearrangement can occur when trying to hydrate an alkene in acid. If we add water and acid (acid catalyzed hyd ...
... (c) Which reagent(s) would you use if you wanted to substitute the alcohol with bromide in the same position and with inversion of configuration, without any rearrangement. (d) This type of rearrangement can occur when trying to hydrate an alkene in acid. If we add water and acid (acid catalyzed hyd ...
chm238f02.exam2
... (c) Which reagent(s) would you use if you wanted to substitute the alcohol with bromide in the same position and with inversion of configuration, without any rearrangement. ...
... (c) Which reagent(s) would you use if you wanted to substitute the alcohol with bromide in the same position and with inversion of configuration, without any rearrangement. ...
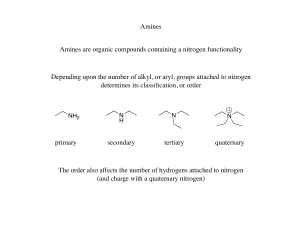
Organic compounds containing Nitrogen
... (iii) Aniline and N-methylamiline. Ans. i) Methylamine and dimethyl amine by isocyanide test: Methyl amine (primary) responds to carbyl amaine reaction. When heated with chloroform and KOH, it produces foul smell. Dimethyl amine (secondary) does not respond to this test. ...
... (iii) Aniline and N-methylamiline. Ans. i) Methylamine and dimethyl amine by isocyanide test: Methyl amine (primary) responds to carbyl amaine reaction. When heated with chloroform and KOH, it produces foul smell. Dimethyl amine (secondary) does not respond to this test. ...
Aminoketone Rearrangements. 11. The Rearrangement of Phenyl a
... absorption a t 5.82 p , indicating the unconjugated carbonyl of IIIa; an absorption band a t 6.05 p , characteristic of ketone imines; and a slight absorption a t 5.93 p corresponding to the conjugated carbonyl of IIa. After this mixture of bases was hydrolyzed with dilute acid, the ketone imine ban ...
... absorption a t 5.82 p , indicating the unconjugated carbonyl of IIIa; an absorption band a t 6.05 p , characteristic of ketone imines; and a slight absorption a t 5.93 p corresponding to the conjugated carbonyl of IIa. After this mixture of bases was hydrolyzed with dilute acid, the ketone imine ban ...
enzymatic And Limited Industrial Use
... A reaction that can proceed in more than one way to produce different products involving different carbon atoms, where one predominates. It is said to be regioselective. ...
... A reaction that can proceed in more than one way to produce different products involving different carbon atoms, where one predominates. It is said to be regioselective. ...
Document
... If it smells of “fish” or “rotting flesh” chances are you have an amine!! Amines behave in a similar way to NH3 but their behaviour is modified by the alkyl groups. 2.1 Bonding: cf NH3 ...
... If it smells of “fish” or “rotting flesh” chances are you have an amine!! Amines behave in a similar way to NH3 but their behaviour is modified by the alkyl groups. 2.1 Bonding: cf NH3 ...
f8560d95306293b
... Reaction of Ethers with Strong Acid • In order for ethers to undergo substitution or elimination reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. • HBr and HI are strong acids that are also sources of good nucleo ...
... Reaction of Ethers with Strong Acid • In order for ethers to undergo substitution or elimination reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. • HBr and HI are strong acids that are also sources of good nucleo ...
22.4: Acidity of Phenols.
... 22.12: Cleavage of Aryl Ethers by Hydrogen Halides. Aryl alkyl ethers can be cleaved by HX to give phenols. ...
... 22.12: Cleavage of Aryl Ethers by Hydrogen Halides. Aryl alkyl ethers can be cleaved by HX to give phenols. ...
Amines - ChemConnections
... stomach and GI pH will decrease the absorption of Adderall,[10] and acidic urine levels will decrease the reabsorption of the drug through the renal system.[11] Co-administration of acidic substances (e.g. citric acid) causes decreased renal reabsorption of DL-amphetamine, while alkaline agents (e.g ...
... stomach and GI pH will decrease the absorption of Adderall,[10] and acidic urine levels will decrease the reabsorption of the drug through the renal system.[11] Co-administration of acidic substances (e.g. citric acid) causes decreased renal reabsorption of DL-amphetamine, while alkaline agents (e.g ...
ppt
... Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.3) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably more acidic than alkyl amines (pKa < 5). The nitrogen lone pair is less basic if it is in an sp2 hybridized ...
... Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.3) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably more acidic than alkyl amines (pKa < 5). The nitrogen lone pair is less basic if it is in an sp2 hybridized ...