MASSACHUSETTS INSTITUTE OF TECHNOLOGY
... following problem are to be based on [ω = 1000, m = 1, x = 10, and kLR = 1] or [ω = 1000, m = 1, x = 1, kLR = 10]. The first set of parameters is close to the normal mode limit and the second set of parameters is close to the local mode limit. A. Set up the 11 × 11 P = 10 polyad. Solve for the energ ...
... following problem are to be based on [ω = 1000, m = 1, x = 10, and kLR = 1] or [ω = 1000, m = 1, x = 1, kLR = 10]. The first set of parameters is close to the normal mode limit and the second set of parameters is close to the local mode limit. A. Set up the 11 × 11 P = 10 polyad. Solve for the energ ...
Glasgow2004
... The main application of MUBs pertains to secure quantum key exchange (quantum cryptography). This is because any attempt by an eavesdropper (say Eve) to distinguish between two nonorthogonal quantum states shared by two remote parties (say Alice and Bob) will occur at the price of introducing a dist ...
... The main application of MUBs pertains to secure quantum key exchange (quantum cryptography). This is because any attempt by an eavesdropper (say Eve) to distinguish between two nonorthogonal quantum states shared by two remote parties (say Alice and Bob) will occur at the price of introducing a dist ...
Harmonic oscillator
... it would be in the classical case. Still, to find a quantum-to-classical correspondence it is not enough to choose a stationary eigenstate of the Hamiltonian with a high energy (high n): this state would still have zero expectation value for the momentum and position. In contrast, the position evolu ...
... it would be in the classical case. Still, to find a quantum-to-classical correspondence it is not enough to choose a stationary eigenstate of the Hamiltonian with a high energy (high n): this state would still have zero expectation value for the momentum and position. In contrast, the position evolu ...
1. Crystal Properties and Growth of Semiconductors
... radiation emanating from them Bohr postulates: 1) Electron exists in certain stable circular orbits about the nucleus and does not give off radiation 2) Electron may shift to an orbit of higher or lower energy by absorbing or emitting a photon of energy hf 3) Angular momentum is quantized p =m v r = ...
... radiation emanating from them Bohr postulates: 1) Electron exists in certain stable circular orbits about the nucleus and does not give off radiation 2) Electron may shift to an orbit of higher or lower energy by absorbing or emitting a photon of energy hf 3) Angular momentum is quantized p =m v r = ...
Baby-Quiz
... don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons depends on the frequency of incident light? ...
... don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons depends on the frequency of incident light? ...
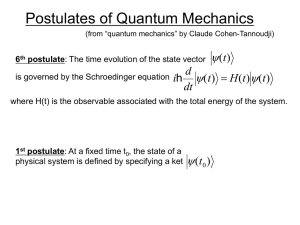
chem6V19_postulates
... 3rd postulate: The only possible result of the measurement of a physical quantity Q is one of the eigenvalues of the corresponding observable ...
... 3rd postulate: The only possible result of the measurement of a physical quantity Q is one of the eigenvalues of the corresponding observable ...
Lecture notes, part 2
... to classical mechanics in some way”! To be able to turn this into a mathematical statement, we shall require that quantum mechanics reproduce classical mechanics “on average”, namely at the level of expectation value. In particular, we have in classical mechanics that px = mv = m ...
... to classical mechanics in some way”! To be able to turn this into a mathematical statement, we shall require that quantum mechanics reproduce classical mechanics “on average”, namely at the level of expectation value. In particular, we have in classical mechanics that px = mv = m ...
Exponential Operator Algebra
... What is the Wave Function of a Swinging Pendulum? Consider a macroscopic simple harmonic oscillator, and to keep things simple assume there are no interactions with the rest of the universe. We know how to describe the motion using classical mechanics: for a given initial position and momentum, clas ...
... What is the Wave Function of a Swinging Pendulum? Consider a macroscopic simple harmonic oscillator, and to keep things simple assume there are no interactions with the rest of the universe. We know how to describe the motion using classical mechanics: for a given initial position and momentum, clas ...
The Heisenberg Uncertainty derivations
... Some other uncertainty relations: 1) In 3-D, (Note: there is no uncertainty relation between, say, observables x and py, nor between, say, x and z, since these pairs commute.) ...
... Some other uncertainty relations: 1) In 3-D, (Note: there is no uncertainty relation between, say, observables x and py, nor between, say, x and z, since these pairs commute.) ...
Thesis Presentation Mr. Joshuah T. Heath Department of Physics
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
Creation and Destruction Operators and Coherent States
... by any system which can be represented in terms of a harmonic oscillator, or sums of harmonic oscillators. They are the answer to the question, what is the state of a quantum oscillator when it is behaving as classically as possible? As a practical example,the state of photons in a laser is quantum ...
... by any system which can be represented in terms of a harmonic oscillator, or sums of harmonic oscillators. They are the answer to the question, what is the state of a quantum oscillator when it is behaving as classically as possible? As a practical example,the state of photons in a laser is quantum ...
Information quantique
... measurement back-action. Quantum computing, where qubits replace classical bits. For some specific algorithms, “quantum parallelism” can actually lead to a fundamentally faster performance than the best known classical algorithms. Quantum simulations, where a wellcontrolled quantum system is designe ...
... measurement back-action. Quantum computing, where qubits replace classical bits. For some specific algorithms, “quantum parallelism” can actually lead to a fundamentally faster performance than the best known classical algorithms. Quantum simulations, where a wellcontrolled quantum system is designe ...
PH5015 - Applications of Quantum Physics
... The second half of the course explores the statistics of light: coherence. First and second order correlation functions. Chaotic light, coherent light. Photon statistics. Sub and super Poissonian light. Photon bunching and antibunching. Quantum cryptography. Entangled states. Single photon sources. ...
... The second half of the course explores the statistics of light: coherence. First and second order correlation functions. Chaotic light, coherent light. Photon statistics. Sub and super Poissonian light. Photon bunching and antibunching. Quantum cryptography. Entangled states. Single photon sources. ...
The Quantum Mechanical Model of the Atom
... An atomic orbital can be visualized as a fuzzy cloud where the electron is most likely to be at a given energy level ...
... An atomic orbital can be visualized as a fuzzy cloud where the electron is most likely to be at a given energy level ...
Quiz 4
... 4. (7 points) An electron in a certain atom is in the n = 2 quantum level. List the possible values of l (and for each l list all values of ml ) that it can have. The angular momentum quantum number l can have integral (i.e. whole number) values from 0 to n − 1. In this case n = 2, so the allowed va ...
... 4. (7 points) An electron in a certain atom is in the n = 2 quantum level. List the possible values of l (and for each l list all values of ml ) that it can have. The angular momentum quantum number l can have integral (i.e. whole number) values from 0 to n − 1. In this case n = 2, so the allowed va ...
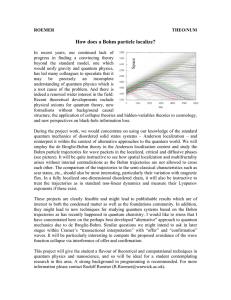
How does a Bohm particle localize?
... employ the de Broglie-Bohm theory in the Anderson localization context and study the Bohm particle trajectories for wave packets in the localized, critical and diffusive phases (see picture). It will be quite instructive to see how spatial localization and multifractality arises without internal con ...
... employ the de Broglie-Bohm theory in the Anderson localization context and study the Bohm particle trajectories for wave packets in the localized, critical and diffusive phases (see picture). It will be quite instructive to see how spatial localization and multifractality arises without internal con ...