Heat is energy transferring in a system and its surroundings.
... Pressure is the force per unit area, where work is also done by a gas when there is a volume change (distance cubed) caused from the gas under pressure. For an isobaric process, the pressure is constant and ...
... Pressure is the force per unit area, where work is also done by a gas when there is a volume change (distance cubed) caused from the gas under pressure. For an isobaric process, the pressure is constant and ...
29-Thermal Exoskeleton - European School Luxembourg
... The first of these involves mapping out the body (or, in the case of a reduced model, general area of interest) in terms of heat sensitive regions to figure out which specific locations the heat would have to be applied to. The second involves understanding the different properties of the materials ...
... The first of these involves mapping out the body (or, in the case of a reduced model, general area of interest) in terms of heat sensitive regions to figure out which specific locations the heat would have to be applied to. The second involves understanding the different properties of the materials ...
Worksheet- Calculations involving Specific Heat
... Worksheet- Calculations involving Specific Heat 1. For q= m ●c ● Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Expla ...
... Worksheet- Calculations involving Specific Heat 1. For q= m ●c ● Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Expla ...
Sample Exam 3
... 9. The term “absolute zero” refers to a) the temperature at which water freezes. b) the temperature at which carbon dioxide freezes. c) the zero point in the Fahrenheit scale. d) the temperature at which all particle motion stops. e) the coldest temperature ever achieved on Earth. ...
... 9. The term “absolute zero” refers to a) the temperature at which water freezes. b) the temperature at which carbon dioxide freezes. c) the zero point in the Fahrenheit scale. d) the temperature at which all particle motion stops. e) the coldest temperature ever achieved on Earth. ...
Heat - Geography1000
... • The assimilation of electromagnetic waves by striking an object. • Different objects have different absorption abilities • Reflection • The ability of an object to repel electromagnetic waves without altering either the object or the waves ...
... • The assimilation of electromagnetic waves by striking an object. • Different objects have different absorption abilities • Reflection • The ability of an object to repel electromagnetic waves without altering either the object or the waves ...
Specific heat
... – Heated solids increase or decrease in all dimensions (length, width, and thickness). – When a solid is heated, the increase in thermal energy increases the average distance between the atoms and molecules of the solid and it expands. ...
... – Heated solids increase or decrease in all dimensions (length, width, and thickness). – When a solid is heated, the increase in thermal energy increases the average distance between the atoms and molecules of the solid and it expands. ...
Heat, Temperature and Atmospheric Circulations
... different parts may be different temperatures ...
... different parts may be different temperatures ...
5.2--FUNSHEET--Heat Temp SHC 6.1 6.2 specific heat capacity
... a difference in their temperatures B. a measure of the average kinetic energy of the particles in a sample of matter C. the energy stored in a sample of matter ...
... a difference in their temperatures B. a measure of the average kinetic energy of the particles in a sample of matter C. the energy stored in a sample of matter ...
Ch 10 Review activity
... water have specific heats of 0.129 J/goC and 4.184 J/goC respectively, what is the final temperature of the mixture? Assume that the gold and the water experience no change in state of matter ...
... water have specific heats of 0.129 J/goC and 4.184 J/goC respectively, what is the final temperature of the mixture? Assume that the gold and the water experience no change in state of matter ...
lec06 - University of Oregon
... Th is the temperature of the hot object Tc is the temperature of the cold object T = Th–Tc is the temperature difference Qh is the amount of heat that flows out of the hot body Qc is the amount of heat flowing into the cold body W is the amount of “useful” mechanical work Sh is th ...
... Th is the temperature of the hot object Tc is the temperature of the cold object T = Th–Tc is the temperature difference Qh is the amount of heat that flows out of the hot body Qc is the amount of heat flowing into the cold body W is the amount of “useful” mechanical work Sh is th ...
Latent Heat
... We know that when we heat the water from 0°C to 100°C, we can calculate how much heat is necessary to add in order to accomplish this by using Q = mcΔT. However, if we plot the heat added to the system against the temperature increase over the entire -40°C to 110°C range, we would not find as linear ...
... We know that when we heat the water from 0°C to 100°C, we can calculate how much heat is necessary to add in order to accomplish this by using Q = mcΔT. However, if we plot the heat added to the system against the temperature increase over the entire -40°C to 110°C range, we would not find as linear ...
Comparison of Heat Loss by Sample Building Component
... Comparison of Heat Loss by Sample Building Component Windows vs. Walls Formula: Heat Loss (BTU/hr) = UA Where U = Thermal transmittance Where A = Area Where = Delta T (temperature difference) Use the formula for calculating heat loss to discuss window replacement as an energy savings measure. Use th ...
... Comparison of Heat Loss by Sample Building Component Windows vs. Walls Formula: Heat Loss (BTU/hr) = UA Where U = Thermal transmittance Where A = Area Where = Delta T (temperature difference) Use the formula for calculating heat loss to discuss window replacement as an energy savings measure. Use th ...
Year 11 General Physics quiz
... If 100J of internal energy is removed from each object (A and B), will they be at the same temperature? No, because they will have a different specific heat capacities. Metal has a lower specific heat value which makes it more sensitive to heat transfer. ...
... If 100J of internal energy is removed from each object (A and B), will they be at the same temperature? No, because they will have a different specific heat capacities. Metal has a lower specific heat value which makes it more sensitive to heat transfer. ...
Quiz-1_MA
... The volume of an iron cube, 5 cm on edge, will increase by what amount if it is heated from 10°C to 60°C? A) ...
... The volume of an iron cube, 5 cm on edge, will increase by what amount if it is heated from 10°C to 60°C? A) ...
Energy
... generated when hydraulic turbines are turned by the force of moving water as it flows through a turbine. ...
... generated when hydraulic turbines are turned by the force of moving water as it flows through a turbine. ...
Key terms in low-temperature insulation
... Convection makes a considerable contribution towards improving the heat transfer coefficient. The faster the ambient air flows,the more heat is transported. In practice, it is therefore essential to ensure that pipes and ducts do not lie too close to each other or at an insufficient distance from wa ...
... Convection makes a considerable contribution towards improving the heat transfer coefficient. The faster the ambient air flows,the more heat is transported. In practice, it is therefore essential to ensure that pipes and ducts do not lie too close to each other or at an insufficient distance from wa ...
SPECIFIC HEAT CAPACITY
... 5. A 4.50 g nugget of gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25°C? The specific heat of gold is 0.129 J/g°C. 6. A 155 g sample of an unknown substance was heated from 25°C to 40°C. In the process, the substance absorbed 5696 J of energy ...
... 5. A 4.50 g nugget of gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25°C? The specific heat of gold is 0.129 J/g°C. 6. A 155 g sample of an unknown substance was heated from 25°C to 40°C. In the process, the substance absorbed 5696 J of energy ...
Tutorial 3
... thick plastic cover whose thermal conductivity is k = 0.15 W/m · °C. Electrical measurements indicate that a current of 10 A passes through the wire and there is a voltage drop of 8 V along the wire. If the insulated wire is exposed to a medium at T = 30°C with a heat transfer coefficient of h = 12 ...
... thick plastic cover whose thermal conductivity is k = 0.15 W/m · °C. Electrical measurements indicate that a current of 10 A passes through the wire and there is a voltage drop of 8 V along the wire. If the insulated wire is exposed to a medium at T = 30°C with a heat transfer coefficient of h = 12 ...
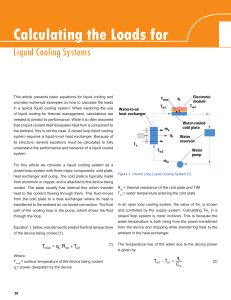
Calculating the Loads for Liquid cooling Systems
... The heat exchanger is often the limiting factor in a compact system due to its relatively large size. Packaging and other mechanical concerns for the heat exchanger dictate that it should be considered first. The cold plate and pump are typically easier to optimize. For example, if a heat exchanger’ ...
... The heat exchanger is often the limiting factor in a compact system due to its relatively large size. Packaging and other mechanical concerns for the heat exchanger dictate that it should be considered first. The cold plate and pump are typically easier to optimize. For example, if a heat exchanger’ ...
Heat Transfer: Conduction, Convection and Latent Heat In addition
... - Heat transfer due to vertical air motions is always referred to as convection - Heat transfer due to horizontal air motions is often referred to more specifically as advection ...
... - Heat transfer due to vertical air motions is always referred to as convection - Heat transfer due to horizontal air motions is often referred to more specifically as advection ...
NOT
... water with an initial temperature of 25 C. The final temperature of the object and water is 29 C. What is the specific heat capacity of the object (Cpw = 4186 J/kg C)? a) 260 J/kg C b) 520 J/kg C c) 129 J/kg C d) 1040 J/kg C ...
... water with an initial temperature of 25 C. The final temperature of the object and water is 29 C. What is the specific heat capacity of the object (Cpw = 4186 J/kg C)? a) 260 J/kg C b) 520 J/kg C c) 129 J/kg C d) 1040 J/kg C ...
ABE 484
... methods. Learn how to interpret the data for the specific need of each problem. Promote critical thinking through problem solving processes. 4. Learn how to set up governing equations, boundary conditions, and initial conditions under various circumstances with different geometries. 5. Use requisite ...
... methods. Learn how to interpret the data for the specific need of each problem. Promote critical thinking through problem solving processes. 4. Learn how to set up governing equations, boundary conditions, and initial conditions under various circumstances with different geometries. 5. Use requisite ...
Chem 30 – Thermochemistry
... C is at a higher energy than A and B - this means that the products have more potential energy than the reactants. This also means C is a less stable compound. Take a look at pages 4-5 of the Chemistry 30 Databook – this is a table of the Standard Molar Enthalpies of Formation. This information tell ...
... C is at a higher energy than A and B - this means that the products have more potential energy than the reactants. This also means C is a less stable compound. Take a look at pages 4-5 of the Chemistry 30 Databook – this is a table of the Standard Molar Enthalpies of Formation. This information tell ...
Heat Transfer: Conduction, Convection and Latent Heat In addition
... warmed by contact with the ground (conduction) and moistened by ...
... warmed by contact with the ground (conduction) and moistened by ...
Cogeneration

Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time. Trigeneration or combined cooling, heat and power (CCHP) refers to the simultaneous generation of electricity and useful heating and cooling from the combustion of a fuel or a solar heat collector. Cogeneration is a thermodynamically efficient use of fuel. In separate production of electricity, some energy must be discarded as waste heat, but in cogeneration this thermal energy is put to use. All thermal power plants emit heat during electricity generation, which can be released into the natural environment through cooling towers, flue gas, or by other means. In contrast, CHP captures some or all of the by-product for heating, either very close to the plant, or—especially in Scandinavia and Eastern Europe—as hot water for district heating with temperatures ranging from approximately 80 to 130 °C. This is also called combined heat and power district heating (CHPDH). Small CHP plants are an example of decentralized energy. By-product heat at moderate temperatures (100–180 °C, 212–356 °F) can also be used in absorption refrigerators for cooling.The supply of high-temperature heat first drives a gas or steam turbine-powered generator and the resulting low-temperature waste heat is then used for water or space heating as described in cogeneration. At smaller scales (typically below 1 MW) a gas engine or diesel engine may be used. Trigeneration differs from cogeneration in that the waste heat is used for both heating and cooling, typically in an absorption refrigerator. CCHP systems can attain higher overall efficiencies than cogeneration or traditional power plants. In the United States, the application of trigeneration in buildings is called building cooling, heating and power (BCHP). Heating and cooling output may operate concurrently or alternately depending on need and system construction.Cogeneration was practiced in some of the earliest installations of electrical generation. Before central stations distributed power, industries generating their own power used exhaust steam for process heating. Large office and apartment buildings, hotels and stores commonly generated their own power and used waste steam for building heat. Due to the high cost of early purchased power, these CHP operations continued for many years after utility electricity became available.