The Laws of Thermodinamics
... energy Qh from the reservoir and does work WAB in raising the piston) • 2. The process B→C , the gas expends adiabatically (energy enter or leave by heat). The temperature falls from Th to Tc, and the gas does work WBC to the raising piston ...
... energy Qh from the reservoir and does work WAB in raising the piston) • 2. The process B→C , the gas expends adiabatically (energy enter or leave by heat). The temperature falls from Th to Tc, and the gas does work WBC to the raising piston ...
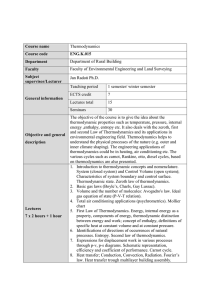
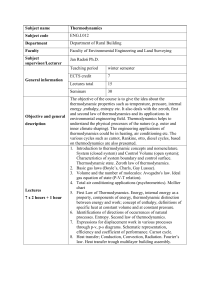
Course name Thermodynamics Course code ENG.I.011 Department
... Faculty of Environmental Engineering and Land Surveying ...
... Faculty of Environmental Engineering and Land Surveying ...
Chapter 10: Simple Harmonic Motion
... Lightning strikes the ground with a current of 100 kA. A person and a cow are each a radial distance D=60.0 m from the lightning strike. The current spreads through the ground uniformly over a hemisphere centered on the strike point. The person’s feet are separated by radial distance rper=0.50 m; t ...
... Lightning strikes the ground with a current of 100 kA. A person and a cow are each a radial distance D=60.0 m from the lightning strike. The current spreads through the ground uniformly over a hemisphere centered on the strike point. The person’s feet are separated by radial distance rper=0.50 m; t ...
electrical heating - Home - KSU Faculty Member websites
... converted to heat. Common applications include heating of buildings, cooking, and industrial processes. An electric heater is an electrical appliance that converts electrical energy into heat . The heating element inside every electric heater is simply an electrical resistor, and works on the princi ...
... converted to heat. Common applications include heating of buildings, cooking, and industrial processes. An electric heater is an electrical appliance that converts electrical energy into heat . The heating element inside every electric heater is simply an electrical resistor, and works on the princi ...
Name: Date: ______ Thermochemistry Round Robin
... 13. The specific heat of silver is 0.235 J/g˚C. Its melting point is 962˚C, and its heat of fusion is 11.3 kJ/mol. What quantity of heat, in joules, is required to change 5.00 g of silver from solid at 25˚C to liquid at 962˚C? ...
... 13. The specific heat of silver is 0.235 J/g˚C. Its melting point is 962˚C, and its heat of fusion is 11.3 kJ/mol. What quantity of heat, in joules, is required to change 5.00 g of silver from solid at 25˚C to liquid at 962˚C? ...
Heat
... 1) One can refer to heat only when energy has been transferred as a result of a temperature difference. Both heat and work are ways of changing the energy of a system. 2) the internal energy of a system can be changed even when no energy is transferred by heat. For example, when a gas in an insulat ...
... 1) One can refer to heat only when energy has been transferred as a result of a temperature difference. Both heat and work are ways of changing the energy of a system. 2) the internal energy of a system can be changed even when no energy is transferred by heat. For example, when a gas in an insulat ...
Latent Heat
... The process also works in reverse: when water changes from steam to water, it releases heat in the same amount. Note that some materials can change from liquid to gas at temperatures other than the boiling point – for example, when water evaporates from your skin. When water evaporates, the latent h ...
... The process also works in reverse: when water changes from steam to water, it releases heat in the same amount. Note that some materials can change from liquid to gas at temperatures other than the boiling point – for example, when water evaporates from your skin. When water evaporates, the latent h ...
SUMMARY
... molecular arrangements and forces of attraction between their molecules. A solid has a definite shape and volume because it has molecules that vibrate in a fixed equilibrium position with strong cohesive forces. A liquid has molecules that have cohesive forces strong enough to give it a definite vol ...
... molecular arrangements and forces of attraction between their molecules. A solid has a definite shape and volume because it has molecules that vibrate in a fixed equilibrium position with strong cohesive forces. A liquid has molecules that have cohesive forces strong enough to give it a definite vol ...
Specific Heat of a Metal
... needed to raise the temperature of one gram of the substance one Celsius degree. Often applied to metallic elements, specific heat can be used as a basis for comparing energy absorption and transfer. ...
... needed to raise the temperature of one gram of the substance one Celsius degree. Often applied to metallic elements, specific heat can be used as a basis for comparing energy absorption and transfer. ...
doc heat conversion
... substance becomes hotter. Similarly, when heat energy if withdrawn from a substance the particles move slower and becomes colder. The Relationship between Heat and Temperature When a substance is heated, its temperature rises. Heat decreases or increases the temperature of a body. For instance, when ...
... substance becomes hotter. Similarly, when heat energy if withdrawn from a substance the particles move slower and becomes colder. The Relationship between Heat and Temperature When a substance is heated, its temperature rises. Heat decreases or increases the temperature of a body. For instance, when ...
Implimenting a Simple Heat Exchanger Unit with
... applications requiring small amounts of cooling in a portable environment. If the application requires heating, another completely separate unit must be included. This paper will discuss a method for creating a heat exchanger comprised of three distinct functional blocks; the two mediums between whi ...
... applications requiring small amounts of cooling in a portable environment. If the application requires heating, another completely separate unit must be included. This paper will discuss a method for creating a heat exchanger comprised of three distinct functional blocks; the two mediums between whi ...
File - El Paso High School
... maintained at a constant value by putting the system in contact with a constant-temperature reservoir (the thermodynamic definition of a reservoir is something large enough that it can transfer heat into or out of a system without changing temperature). - An example of an adiabatic process is a gas ...
... maintained at a constant value by putting the system in contact with a constant-temperature reservoir (the thermodynamic definition of a reservoir is something large enough that it can transfer heat into or out of a system without changing temperature). - An example of an adiabatic process is a gas ...
heat
... When heat flows from hot to cold, the molecules end up all having the same temperature and kinetic energy (less order). To flow from cold to hot, work has to be done because that direction would produce more order. Engines cannot produce the same amount of work as the initial heat because some of th ...
... When heat flows from hot to cold, the molecules end up all having the same temperature and kinetic energy (less order). To flow from cold to hot, work has to be done because that direction would produce more order. Engines cannot produce the same amount of work as the initial heat because some of th ...
An Approach to a Zero
... electricity usage might be about 400 kWh for everything (lights, fridge, washer, electric dryer). This is an average of about 550 W. For a zero-energy house, it is reasonable to expect to use the most efficient lamps and appliances. Maybe we can get down to 400 W on average. Covering 100 m2 with pho ...
... electricity usage might be about 400 kWh for everything (lights, fridge, washer, electric dryer). This is an average of about 550 W. For a zero-energy house, it is reasonable to expect to use the most efficient lamps and appliances. Maybe we can get down to 400 W on average. Covering 100 m2 with pho ...
Thermo-regulation - Learning Central
... The rate at which this heat is produced (the metabolic rate or MR) is measured in kilocalories. Resting metabolic rate varies with age and sex. ...
... The rate at which this heat is produced (the metabolic rate or MR) is measured in kilocalories. Resting metabolic rate varies with age and sex. ...