Chemistry Final Exam Test Yourself I
... (Concentration, surface area, temperature, and adding a catalyst) As the number of ions increases in a solution, the ____________ goes down ...
... (Concentration, surface area, temperature, and adding a catalyst) As the number of ions increases in a solution, the ____________ goes down ...
precipitation rxn_level_packet
... Directions for the following 4 reactions: a. In one well of a well-plate, add three drops of each substance. b. Write down your observations for the reactants above. c. In parenthesis provided above, indicate if the product is soluble with an “aq” or forms a precipitate (solid) with an “s.” 1. Write ...
... Directions for the following 4 reactions: a. In one well of a well-plate, add three drops of each substance. b. Write down your observations for the reactants above. c. In parenthesis provided above, indicate if the product is soluble with an “aq” or forms a precipitate (solid) with an “s.” 1. Write ...
chapters 16-17 test re
... the activated complex, and the reaction rate is fast. 4. _______ Catalysts are enzymes that aren’t consumed in a chemical reaction, but they raise the reaction rate by lowering the Ea. 5. _______ To calculate the overall reaction rate add the exponents of the reactants together. 6. _______ Intermedi ...
... the activated complex, and the reaction rate is fast. 4. _______ Catalysts are enzymes that aren’t consumed in a chemical reaction, but they raise the reaction rate by lowering the Ea. 5. _______ To calculate the overall reaction rate add the exponents of the reactants together. 6. _______ Intermedi ...
Practice Final Exam, Chemistry 2220, Organic Chem II 1. Rank the
... 6. Which one of the following compounds is NOT a product of reaction between 1,3butadiene and HBr? A. (S)-3-bromo-1-butene B. (R)-3-bromo-1-butene C. (Z)-2-bromo-2-butene D. (E)-1-bromo-2-butene 7. Choose the reagents necessary to carry out the following conversion. O ...
... 6. Which one of the following compounds is NOT a product of reaction between 1,3butadiene and HBr? A. (S)-3-bromo-1-butene B. (R)-3-bromo-1-butene C. (Z)-2-bromo-2-butene D. (E)-1-bromo-2-butene 7. Choose the reagents necessary to carry out the following conversion. O ...
Branches of Chemistry
... Analytical chemists explore the types and proportions of substances in a sample. Astrochemists identify substances found in stars and other bodies in space. Biochemists study the compounds and chemical reactions in living organisms. Electrochemists investigate the relationship between the flow of el ...
... Analytical chemists explore the types and proportions of substances in a sample. Astrochemists identify substances found in stars and other bodies in space. Biochemists study the compounds and chemical reactions in living organisms. Electrochemists investigate the relationship between the flow of el ...
AP Chemistry Syllabus 2013 Mawhiney
... Labs form a foundation for student understanding of the chemical principles discussed in lectures but are also chosen to reflect the diversity of lab work generally completed in a first year course. Analysis of data from AP Chemistry examinees shows that increased laboratory time is correlated with ...
... Labs form a foundation for student understanding of the chemical principles discussed in lectures but are also chosen to reflect the diversity of lab work generally completed in a first year course. Analysis of data from AP Chemistry examinees shows that increased laboratory time is correlated with ...
Ahmed Fazary_Click Chemistry
... Click chemistry is a concept introduced by K. Barry Sharpless in 2001 and describes chemistry tailored to generate substances quickly and reliably by joining small units together as nature does. In biochemistry, proteins are made from repeating amino acid units and sugars are made from repeating mon ...
... Click chemistry is a concept introduced by K. Barry Sharpless in 2001 and describes chemistry tailored to generate substances quickly and reliably by joining small units together as nature does. In biochemistry, proteins are made from repeating amino acid units and sugars are made from repeating mon ...
General Chemistry First Semester Review General
... valence electrons and how they are arranged to satisfy the “octet rule”). 8. What kind of bonds (ionic or covalent) are most likely holding a particle of magnesium chloride? How do you know? 9. How many valence electrons does an atom of silicon possess? 10. Are metals more likely to accept or donate ...
... valence electrons and how they are arranged to satisfy the “octet rule”). 8. What kind of bonds (ionic or covalent) are most likely holding a particle of magnesium chloride? How do you know? 9. How many valence electrons does an atom of silicon possess? 10. Are metals more likely to accept or donate ...
Chemistry - Target Publications
... Answers to the two sections are to be written in the same answer book. iii. Figures to the right hand side indicate full marks. iv. Write balanced chemical equations and draw neat and labelled diagrams, wherever necessary. v. Use of logarithmic table is allowed. vi. Answer to every question must be ...
... Answers to the two sections are to be written in the same answer book. iii. Figures to the right hand side indicate full marks. iv. Write balanced chemical equations and draw neat and labelled diagrams, wherever necessary. v. Use of logarithmic table is allowed. vi. Answer to every question must be ...
RXN-4-STUDENTS - Rothschild Science
... different molecules switch places, forming two entirely different compounds ...
... different molecules switch places, forming two entirely different compounds ...
Basic Background Review: Acid-Base , Redox, and Stable Isotopes
... IE actual carbon Std Ratio 12C/ 13C = already a BIG excess Boils down to measuring absolute differences in a small populations of atoms of the Heavy Isotope. (Analogy: being able to measure relative differences in distance of a cm or two, accurately and reproducibly of the scale of here to ...
... IE actual carbon Std Ratio 12C/ 13C = already a BIG excess Boils down to measuring absolute differences in a small populations of atoms of the Heavy Isotope. (Analogy: being able to measure relative differences in distance of a cm or two, accurately and reproducibly of the scale of here to ...
Chemical reactions unit
... 4. Increase in pressure: Why? Particles are squeezed into a smaller volume, so there is less space and more collisions occur between particles. ...
... 4. Increase in pressure: Why? Particles are squeezed into a smaller volume, so there is less space and more collisions occur between particles. ...
Chemical reactions unit
... 4. Increase in pressure: Why? Particles are squeezed into a smaller volume, so there is less space and more collisions occur between particles. ...
... 4. Increase in pressure: Why? Particles are squeezed into a smaller volume, so there is less space and more collisions occur between particles. ...
inorganic-chemistry-gp-i-alkali-metals
... The colour of the superoxide’s is due to the paramagnetic behaviour, the O2- is having two covalent bonds and a single electron, which when move from one to other atom releases photon of visible range giving the compounds colour, and also the paramagnetic behaviour The stability of peroxides and s ...
... The colour of the superoxide’s is due to the paramagnetic behaviour, the O2- is having two covalent bonds and a single electron, which when move from one to other atom releases photon of visible range giving the compounds colour, and also the paramagnetic behaviour The stability of peroxides and s ...
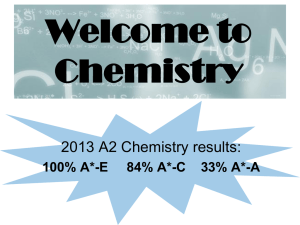
Welcome to Chemistry
... A numerate subject such as CHEMISTRY is useful for… • Accountancy/Business • Architecture • Law ...
... A numerate subject such as CHEMISTRY is useful for… • Accountancy/Business • Architecture • Law ...
Chemistry FINAL: CONTENT Review Packet
... 9. Which of the 4 phases of matter is considered to have the most significant intermolecular forces? ________ a) The least significant intermolecular forces? _______________ 10. A liquid will boil when its equilibrium vapor pressure EQUALS____________________________. 11. When a system at equilibriu ...
... 9. Which of the 4 phases of matter is considered to have the most significant intermolecular forces? ________ a) The least significant intermolecular forces? _______________ 10. A liquid will boil when its equilibrium vapor pressure EQUALS____________________________. 11. When a system at equilibriu ...
Structure-activity relationships
... while maternal deaths from infections arising during childbirth declined by more than 90%. Illnesses such as tuberculosis, diphtheria, and pneumonia could be treated and cured for the first time in human history. ...
... while maternal deaths from infections arising during childbirth declined by more than 90%. Illnesses such as tuberculosis, diphtheria, and pneumonia could be treated and cured for the first time in human history. ...
CHEMISTRY
... (3) Amphoteric compounds (4) Lewis acids 34. An alkane has a C/H ratio (by mass) of 5.1428. Its molecular formula is: ...
... (3) Amphoteric compounds (4) Lewis acids 34. An alkane has a C/H ratio (by mass) of 5.1428. Its molecular formula is: ...