HALO-ORGANICS – Fully functional fluorine 1
... Fluorine is a common element in antibiotic molecules, too. One of the biggest sellers in recent years – Bayer’s Cipro (ciprofloxacin) has an aromatic fluorine substituent, as has norfloxaxin (Noroxin from Dainippon) and levofloxacin (Levoquin), Ortho McNeil’s single isomer version of the older drug ...
... Fluorine is a common element in antibiotic molecules, too. One of the biggest sellers in recent years – Bayer’s Cipro (ciprofloxacin) has an aromatic fluorine substituent, as has norfloxaxin (Noroxin from Dainippon) and levofloxacin (Levoquin), Ortho McNeil’s single isomer version of the older drug ...
Chapter 13, sections 13.5 - Properties of Aldehydes and Ketones
... OH group. • An aldehyde is oxidized to a carboxylic acid, while Cu2+ is reduced to give red Cu2O(s). ...
... OH group. • An aldehyde is oxidized to a carboxylic acid, while Cu2+ is reduced to give red Cu2O(s). ...
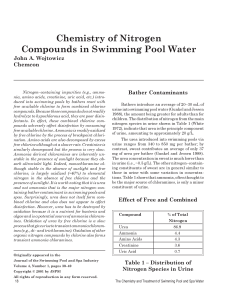
w_4-3 Chemistry of Nitrogen Compounds
... For the theoretical r value of 1.5, the chlorine dose is 7.6 ppm/ppm ammonia nitrogen and for the typical actual r value of 1.75, the chlorine dose is 8.9 ppm/ppm ammonia nitrogen. However, ammonia itself is typically not present in a chlorine treated pool, but rather in the form of a chloramine. If ...
... For the theoretical r value of 1.5, the chlorine dose is 7.6 ppm/ppm ammonia nitrogen and for the typical actual r value of 1.75, the chlorine dose is 8.9 ppm/ppm ammonia nitrogen. However, ammonia itself is typically not present in a chlorine treated pool, but rather in the form of a chloramine. If ...
Summer Study Assignment – Honors Chem 2/AP Chemistry
... 104. An essential amino acid which cannot be made (synthesized) by the body and must be obtained in the diet is methionine. What is the percentage of carbon, nitrogen, and sulfur in this amino acid if the formula of methionine is CH3SCH2CH2CHNH2COOH? 105. Ammonia is produced by the reaction of nitr ...
... 104. An essential amino acid which cannot be made (synthesized) by the body and must be obtained in the diet is methionine. What is the percentage of carbon, nitrogen, and sulfur in this amino acid if the formula of methionine is CH3SCH2CH2CHNH2COOH? 105. Ammonia is produced by the reaction of nitr ...
Chemistry - An Introduction for Medical and Hea..
... of aspirin-like medicines in Britain. It cured the pains from various complaints. Herbal concoctions have been the basis of healing and also poisoning for centuries. Curare was used on the tips of poison darts to kill opponents, but in smaller quantities it was used as a muscle relaxant in surgery u ...
... of aspirin-like medicines in Britain. It cured the pains from various complaints. Herbal concoctions have been the basis of healing and also poisoning for centuries. Curare was used on the tips of poison darts to kill opponents, but in smaller quantities it was used as a muscle relaxant in surgery u ...
Chemistry: An Introduction for Medical and Health Sciences - E
... of aspirin-like medicines in Britain. It cured the pains from various complaints. Herbal concoctions have been the basis of healing and also poisoning for centuries. Curare was used on the tips of poison darts to kill opponents, but in smaller quantities it was used as a muscle relaxant in surgery u ...
... of aspirin-like medicines in Britain. It cured the pains from various complaints. Herbal concoctions have been the basis of healing and also poisoning for centuries. Curare was used on the tips of poison darts to kill opponents, but in smaller quantities it was used as a muscle relaxant in surgery u ...
Prep UK-intro.p65
... - carbonium ions in addition reaction - relative stability of carbonium ions ...
... - carbonium ions in addition reaction - relative stability of carbonium ions ...
THE STUDY OF INTERMEDIARY METABOLISM OF
... atoms. Many compounds when treated with hot concentrated DzSO4 exchange otherwise stable hydrogen atoms (59). A number of deuterium-containing fatty acids and amino acids have thus been prepared by this procedure (60, 61). The method introduces deuterium into fatty acids only at the a-carbon atom. A ...
... atoms. Many compounds when treated with hot concentrated DzSO4 exchange otherwise stable hydrogen atoms (59). A number of deuterium-containing fatty acids and amino acids have thus been prepared by this procedure (60, 61). The method introduces deuterium into fatty acids only at the a-carbon atom. A ...
Cycloalkanes - faculty at Chemeketa
... conformation of cyclohexane when all bonds rotate in a synchronized fashion? ...
... conformation of cyclohexane when all bonds rotate in a synchronized fashion? ...
Evaluation of constructed wetlands for treating hydroponic waste
... To treat the hydroponic waste solution containing high nitrate in constructed wetlands (CWs), the optimum conditions of Thiobacillus denitrificans (sulfur oxidizing denitrifying bacteria) were investigated in batch experiments under various conditions (amount of sulfur, the ratios of sulfur to calci ...
... To treat the hydroponic waste solution containing high nitrate in constructed wetlands (CWs), the optimum conditions of Thiobacillus denitrificans (sulfur oxidizing denitrifying bacteria) were investigated in batch experiments under various conditions (amount of sulfur, the ratios of sulfur to calci ...
covalent - Typepad
... 52. In which of these compounds is the bond between the atoms NOT a nonpolar covalent bond? a. Cl2 c. HCl b. H2 d. O2 53. To draw a Lewis structure, it is NOT necessary to know a. bond energies. b. the types of atoms in the molecule. c. the number of valence electrons for each atom. d. the number of ...
... 52. In which of these compounds is the bond between the atoms NOT a nonpolar covalent bond? a. Cl2 c. HCl b. H2 d. O2 53. To draw a Lewis structure, it is NOT necessary to know a. bond energies. b. the types of atoms in the molecule. c. the number of valence electrons for each atom. d. the number of ...
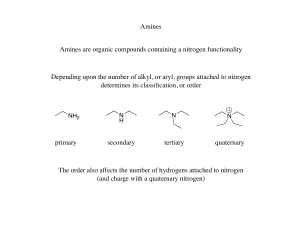
Amines
... none bonding electrons at nitrogen atom to form bond with an acid. • The more easier the lone pair electrons formed bond with the acid, will make the amines a stronger base. • Factors that effect the basicity of the amines: i) substitution by alkyl groups - the presence of alkyl groups (electron-don ...
... none bonding electrons at nitrogen atom to form bond with an acid. • The more easier the lone pair electrons formed bond with the acid, will make the amines a stronger base. • Factors that effect the basicity of the amines: i) substitution by alkyl groups - the presence of alkyl groups (electron-don ...
Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... ¾ HCl, Hydrogen Chloride as a molecule becomes hydrochloric acid Oxyacids are acids that contain Hydrogen, oxygen and another element (which is the central element. To write the molecular formula, H first, then central element, then Oxygen ie., HNO3 → Nitric Acid; H2CO3 → Carbonic Acid; H2SO4 → Sulp ...
... ¾ HCl, Hydrogen Chloride as a molecule becomes hydrochloric acid Oxyacids are acids that contain Hydrogen, oxygen and another element (which is the central element. To write the molecular formula, H first, then central element, then Oxygen ie., HNO3 → Nitric Acid; H2CO3 → Carbonic Acid; H2SO4 → Sulp ...
ordinary level chemistry syllabus
... 1.2.1. Chemistry and society Chemistry, one of the natural science subjects, is an important discipline that has contributed significantly to the global socio-economic transformation. This level of contribution has been achieved through the range of important life changing discoveries by chemists. T ...
... 1.2.1. Chemistry and society Chemistry, one of the natural science subjects, is an important discipline that has contributed significantly to the global socio-economic transformation. This level of contribution has been achieved through the range of important life changing discoveries by chemists. T ...
Chapter 3
... atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it is huge number is not?!!!! It is clear that we can not weight a single atom, but as ...
... atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it is huge number is not?!!!! It is clear that we can not weight a single atom, but as ...
102 Lecture Ch19
... and so have high melting and boiling points • Tertiary amides can’t H-bond with themselves, so have lower melting and boiling points than primary or secondary • Amides have higher melting and boiling points than carboxylic acids because they are more flat (due to delocalization of electrons), so the ...
... and so have high melting and boiling points • Tertiary amides can’t H-bond with themselves, so have lower melting and boiling points than primary or secondary • Amides have higher melting and boiling points than carboxylic acids because they are more flat (due to delocalization of electrons), so the ...
Pharmacognosy-I (Part-7)
... atoms (usually in a heterocyclic ring) and they usually have a marked physiological action on man or other animals. • The name 'proto-alkaloid' or 'amino-alkaloid' is sometimes applied to compounds such as hordenine, ephedrine and colchicine which lack one or more of the properties of typical alkalo ...
... atoms (usually in a heterocyclic ring) and they usually have a marked physiological action on man or other animals. • The name 'proto-alkaloid' or 'amino-alkaloid' is sometimes applied to compounds such as hordenine, ephedrine and colchicine which lack one or more of the properties of typical alkalo ...
Chapter 19
... Thus the sp2 hybridized amine is harder to protonate (and form a positive charge) ...
... Thus the sp2 hybridized amine is harder to protonate (and form a positive charge) ...
Stoichiometry and the Mole
... • for a gas the units will be g / L • We can determine the density of any gas at STP if we know its formula. • To find the density we need the mass and the volume. • If you assume you have 1 mole than the mass is the molar mass (PT) • At STP the volume is 22.4 L. ...
... • for a gas the units will be g / L • We can determine the density of any gas at STP if we know its formula. • To find the density we need the mass and the volume. • If you assume you have 1 mole than the mass is the molar mass (PT) • At STP the volume is 22.4 L. ...
chapter 5: nomenclature
... The next alkane contains three carbons, the next one four carbons, the next one five carbons, and so on. The names arise directly from the number of carbons. A couple of important points to note: All the names end in ane - thus the alkanes. There is no other way around this-you must learn the name ...
... The next alkane contains three carbons, the next one four carbons, the next one five carbons, and so on. The names arise directly from the number of carbons. A couple of important points to note: All the names end in ane - thus the alkanes. There is no other way around this-you must learn the name ...
IB Chemistry HL Topic5 Questions 1. Which combination of ionic
... Calculate the standard free energy change at 298 K, GӨ, for the reaction in part (a). Use your answer and relevant information from part (d). If you did not obtain an answer to part (a), use SӨ = –360 J K–1 (this is not the correct value). ...
... Calculate the standard free energy change at 298 K, GӨ, for the reaction in part (a). Use your answer and relevant information from part (d). If you did not obtain an answer to part (a), use SӨ = –360 J K–1 (this is not the correct value). ...
chemistry
... 1.3.3 Natural/Man-made Many substances freely used these days are not available from natural sources, but this distinction is not at all useful for chemists, because it tells us little or nothing about the properties of the substance. Many natural substances can be man-made and samples from each sou ...
... 1.3.3 Natural/Man-made Many substances freely used these days are not available from natural sources, but this distinction is not at all useful for chemists, because it tells us little or nothing about the properties of the substance. Many natural substances can be man-made and samples from each sou ...
enthalpy changes
... The amount of energy released from a chemical reaction is affected to the number of moles of reactant or product. If given the mass of reactant or product, the enthalpy change (∆H in kJ) ...
... The amount of energy released from a chemical reaction is affected to the number of moles of reactant or product. If given the mass of reactant or product, the enthalpy change (∆H in kJ) ...
04_01_03.html
... Overview of Chapter This chapter introduces chemical reactions and their mechanisms by focusing on two reactions that yield alkyl halides. (1) alcohol + hydrogen halide ROH + HX RX + H2O (2) alkane + halogen RH + X2 RX + HX Both are substitution reactions ...
... Overview of Chapter This chapter introduces chemical reactions and their mechanisms by focusing on two reactions that yield alkyl halides. (1) alcohol + hydrogen halide ROH + HX RX + H2O (2) alkane + halogen RH + X2 RX + HX Both are substitution reactions ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.