Net Ionic Equation Powerpoint Tutorial
... might occur: the formation of a weak acid. An acid is a compound that has an H+ ion bonded to some negative ion: HNO3 for example is nitric acid. HF is hydrofluoric acid. All acids fall into one of two categories: strong acids and weak acids. A strong acid is one that dissociates 100% in water. That ...
... might occur: the formation of a weak acid. An acid is a compound that has an H+ ion bonded to some negative ion: HNO3 for example is nitric acid. HF is hydrofluoric acid. All acids fall into one of two categories: strong acids and weak acids. A strong acid is one that dissociates 100% in water. That ...
Chapter 25 Alt Notes 0910
... Alkynes contain CC bonds. The simplest alkyne is C2H2, ethyne, or acetylene. Alkynes with only one C C bond have the formula CnH2n-2. Each carbon atom in a C C bond is sp hybridized. Each sp hybrid contains two bonds and two p bonds. The carbon atom will have one single bond and o ...
... Alkynes contain CC bonds. The simplest alkyne is C2H2, ethyne, or acetylene. Alkynes with only one C C bond have the formula CnH2n-2. Each carbon atom in a C C bond is sp hybridized. Each sp hybrid contains two bonds and two p bonds. The carbon atom will have one single bond and o ...
AP Chemistry - Freehold Regional High School District
... The AP Chemistry course is designed to be the equivalent of the general Chemistry course usually taken during the first college year. For some students, this course enables them to undertake, as freshmen, second year work in the Chemistry sequence at their institution or to register in courses in ot ...
... The AP Chemistry course is designed to be the equivalent of the general Chemistry course usually taken during the first college year. For some students, this course enables them to undertake, as freshmen, second year work in the Chemistry sequence at their institution or to register in courses in ot ...
Unit 13: Organic Chemistry
... Regents Practice Problems-Carbon Compounds (ungraded): 1) Which compound must be present in an organic compound? a) Carbon ...
... Regents Practice Problems-Carbon Compounds (ungraded): 1) Which compound must be present in an organic compound? a) Carbon ...
08272012BC Science Chem 12 Chapter 1 Answer Key
... 2. There is a common misconception that a significant increase in the volume of water will occur as water is formed in a reaction that occurs in aqueous solution. This is, of course, nonsense! As the entire reaction occurs in the solvent water, there will simply be a small amount of water formed, re ...
... 2. There is a common misconception that a significant increase in the volume of water will occur as water is formed in a reaction that occurs in aqueous solution. This is, of course, nonsense! As the entire reaction occurs in the solvent water, there will simply be a small amount of water formed, re ...
Answers - Pearson-Global
... pairs of electrons around one of the atoms – in other words, it is nothing like a noble gas structure. Despite the impression often given at GCSE, such compounds are very common – although in the great majority of cases, there are more than 8 electrons around one atom rather than fewer. Students mig ...
... pairs of electrons around one of the atoms – in other words, it is nothing like a noble gas structure. Despite the impression often given at GCSE, such compounds are very common – although in the great majority of cases, there are more than 8 electrons around one atom rather than fewer. Students mig ...
Unit 13: Organic Chemistry
... Regents Practice Problems-Carbon Compounds (ungraded): 1) Which compound must be present in an organic compound? a) Carbon ...
... Regents Practice Problems-Carbon Compounds (ungraded): 1) Which compound must be present in an organic compound? a) Carbon ...
Organic Chemistry II Introduction
... • Important solvents and intermediates • Phenols contain an OH group connected to a carbon in a benzene ring • Methanol, CH3OH, common name is methyl alcohol, a solvent, a fuel additive, produced in large quantities • Ethanol, CH3CH2OH, common name is ethyl alcohol, a solvent, fuel, beverage, produc ...
... • Important solvents and intermediates • Phenols contain an OH group connected to a carbon in a benzene ring • Methanol, CH3OH, common name is methyl alcohol, a solvent, a fuel additive, produced in large quantities • Ethanol, CH3CH2OH, common name is ethyl alcohol, a solvent, fuel, beverage, produc ...
ap 2005 chemistry_b scoring guidelines - AP Central
... Write the formulas to show the reactants and the products for any FIVE of the laboratory situations described below. Answers to more than five choices will not be graded. In all cases, a reaction occurs. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solution a ...
... Write the formulas to show the reactants and the products for any FIVE of the laboratory situations described below. Answers to more than five choices will not be graded. In all cases, a reaction occurs. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solution a ...
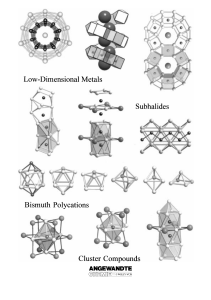
From the Metal to the Molecule
... significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research mainly concerns the ternary and quaternary oxides or halides of the aforementioned main group elements which contain electron-rich transition metals from Groups 8 ± 10 as fur ...
... significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research mainly concerns the ternary and quaternary oxides or halides of the aforementioned main group elements which contain electron-rich transition metals from Groups 8 ± 10 as fur ...
Ch 3
... Vitamin C (M=176.12g/mol) is a compound of C,H, and O found in many natural sources especially citrus fruits. When a 1.000-g sample of vitamin C is placed in a combustion chamber and burned, the following data are obtained: mass of CO2 absorber after combustion ...
... Vitamin C (M=176.12g/mol) is a compound of C,H, and O found in many natural sources especially citrus fruits. When a 1.000-g sample of vitamin C is placed in a combustion chamber and burned, the following data are obtained: mass of CO2 absorber after combustion ...
Learning Outcomes
... to deduce their properties (c) compare the bonding and structures of diamond and graphite in order to deduce their properties such as electrical conductivity, lubricating or cutting action (candidates will not be required to draw the structures) ...................................................... ...
... to deduce their properties (c) compare the bonding and structures of diamond and graphite in order to deduce their properties such as electrical conductivity, lubricating or cutting action (candidates will not be required to draw the structures) ...................................................... ...
Limitations in Determining Enantiomeric Excess of Alcohols by 31P
... primary alcohols have always been recognized as difficult substrates to be discriminated by chiral chromatography, but Feringa reported the successful application of his method to 2-phenyl-1-butanol2. Thus, primary alcohols (±)-3 and (±)-4 (Figs. 3a and 3b), showing no separation in the available ch ...
... primary alcohols have always been recognized as difficult substrates to be discriminated by chiral chromatography, but Feringa reported the successful application of his method to 2-phenyl-1-butanol2. Thus, primary alcohols (±)-3 and (±)-4 (Figs. 3a and 3b), showing no separation in the available ch ...
Chapter 18: Organic Chemistry
... 1. Select the longest carbon chain and name it as the normal straight-chain alkane (this is the "parent chain") 2. Number the parent chain starting at the end of the chain nearest the first alkyl substituent. 3. Use the numbers obtained by the application of rule #2 to designate the locations of the ...
... 1. Select the longest carbon chain and name it as the normal straight-chain alkane (this is the "parent chain") 2. Number the parent chain starting at the end of the chain nearest the first alkyl substituent. 3. Use the numbers obtained by the application of rule #2 to designate the locations of the ...
Forward
... for various resins and plastics. Methanol is also used as a solvent, as an antifreeze, and as a convenient clean-burning liquid fuel. This last property makes it a candidate as a fuel for automobiles—methanol is already used to power Indianapolis-class race cars— but extensive emissions tests remain ...
... for various resins and plastics. Methanol is also used as a solvent, as an antifreeze, and as a convenient clean-burning liquid fuel. This last property makes it a candidate as a fuel for automobiles—methanol is already used to power Indianapolis-class race cars— but extensive emissions tests remain ...
Chemical Reaction Equations
... Limiting and Excess Reagents When no further changes appear to be occurring, we assume that all of the AgNO3(aq) that was initially present has now been completely reacted. A limiting reagent is the reactant whose entities are completely consumed in a reaction, meaning the reaction stops. In order ...
... Limiting and Excess Reagents When no further changes appear to be occurring, we assume that all of the AgNO3(aq) that was initially present has now been completely reacted. A limiting reagent is the reactant whose entities are completely consumed in a reaction, meaning the reaction stops. In order ...
Isomerism - Knockhardy
... another form of stereoisomerism occurs when compounds have non-superimposable mirror images ...
... another form of stereoisomerism occurs when compounds have non-superimposable mirror images ...
No Slide Title
... another form of stereoisomerism occurs when compounds have non-superimposable mirror images ...
... another form of stereoisomerism occurs when compounds have non-superimposable mirror images ...
CHAPTER 15
... type contain more alkoxy groups than the second listed compound type? a) acetal, hemiacetal b) hemiacetal, acetal c) aldehyde, hemiacetal d) more than one correct response e) no correct response ...
... type contain more alkoxy groups than the second listed compound type? a) acetal, hemiacetal b) hemiacetal, acetal c) aldehyde, hemiacetal d) more than one correct response e) no correct response ...
aldehydes and ketones
... Lower members of aldehydes and ketones ( upto C4) are soluble in water due to H-bonding between polar carbonyl group and water. However, solubility decreases with increase in molecular weight Aromatic aldehydes and ketones are much less soluble than corresponding aliphatic aldehydes and ketone ...
... Lower members of aldehydes and ketones ( upto C4) are soluble in water due to H-bonding between polar carbonyl group and water. However, solubility decreases with increase in molecular weight Aromatic aldehydes and ketones are much less soluble than corresponding aliphatic aldehydes and ketone ...
Stoichiometry PP
... 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. ...
... 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. ...
H/ t = W/m
... Since the nitrates can not exist by themselves, nitrate must combine with water vapor to form nitric acid, hence giving the overall reaction: 2N2 + 5O2 + 2H2O = 4HNO3. Cysteine and cystine both contain approximately 26.7% sulfur. The presence of this element in our experimental compounds creates sec ...
... Since the nitrates can not exist by themselves, nitrate must combine with water vapor to form nitric acid, hence giving the overall reaction: 2N2 + 5O2 + 2H2O = 4HNO3. Cysteine and cystine both contain approximately 26.7% sulfur. The presence of this element in our experimental compounds creates sec ...
Learning Guide for Chapter 16
... What does the starting material look like before the H is removed to make the alkoxide? What is this functional group called? ...
... What does the starting material look like before the H is removed to make the alkoxide? What is this functional group called? ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.