Unit 2: Minerals
... • Determine the mass of the mineral • Suspend the mineral in a beaker of water and redetermine its mass • Find how much mass was lost when the mineral was suspended in water. • Divide the mass in air by the amount of mass lost when suspended in water ...
... • Determine the mass of the mineral • Suspend the mineral in a beaker of water and redetermine its mass • Find how much mass was lost when the mineral was suspended in water. • Divide the mass in air by the amount of mass lost when suspended in water ...
Mineral Lab
... environment. In order to comprehend and explain certain aspects of geology, it is necessary to be familiar with the characteristics of minerals and rocks. Minerals exhibit a number of diagnostic properties that are used for identification. A mineral is a naturally occurring inorganic substance with ...
... environment. In order to comprehend and explain certain aspects of geology, it is necessary to be familiar with the characteristics of minerals and rocks. Minerals exhibit a number of diagnostic properties that are used for identification. A mineral is a naturally occurring inorganic substance with ...
Metamorphic Rocks Examples - Uplift Community High School
... events when shales or clay rich sandstones (wackes), or felsic igneous rocks (e.g. granite, granodiorite, etc.) are metamorphosed through depth of burial, and proximity to ...
... events when shales or clay rich sandstones (wackes), or felsic igneous rocks (e.g. granite, granodiorite, etc.) are metamorphosed through depth of burial, and proximity to ...
02 Chapter 2_Matter and Minerals
... of dilute hydrochloric acid on a freshly broken mineral surface. Using this technique, certain minerals, called carbonates, will effervesce يفور (fizz) as carbon dioxide gas is released Calcite reacting with a weak acid ...
... of dilute hydrochloric acid on a freshly broken mineral surface. Using this technique, certain minerals, called carbonates, will effervesce يفور (fizz) as carbon dioxide gas is released Calcite reacting with a weak acid ...
MULTIPLE CHOICE: 1. Each element is defined by the number of a
... Calcite and dolomite are: a. important energy resources b. common rock-forming carbonate minerals c. oxide minerals of great value d. ferromagnesian silicates possessing a distinctive sheet structure e. minerals used in the manufacture of pencil leads The chemical formula for olivine is (Mg, Fe)2SiO ...
... Calcite and dolomite are: a. important energy resources b. common rock-forming carbonate minerals c. oxide minerals of great value d. ferromagnesian silicates possessing a distinctive sheet structure e. minerals used in the manufacture of pencil leads The chemical formula for olivine is (Mg, Fe)2SiO ...
1. The atomic number of an atom is based upon the number of
... Calcite and dolomite are: a. important energy resources b. common rock-forming carbonate minerals c. oxide minerals of great value d. ferromagnesian silicates possessing a distinctive sheet structure e. minerals used in the manufacture of pencil leads The chemical formula for olivine is (Mg, Fe)2SiO ...
... Calcite and dolomite are: a. important energy resources b. common rock-forming carbonate minerals c. oxide minerals of great value d. ferromagnesian silicates possessing a distinctive sheet structure e. minerals used in the manufacture of pencil leads The chemical formula for olivine is (Mg, Fe)2SiO ...
Oxides and Hydroxides
... Ore Minerals • It is harder to obtain metals from oxides than from sulfides, because metal-oxygen bonds are stronger • Some elements prefer to bond to oxygen, and do not occur as sulfides, so the ores are oxides ...
... Ore Minerals • It is harder to obtain metals from oxides than from sulfides, because metal-oxygen bonds are stronger • Some elements prefer to bond to oxygen, and do not occur as sulfides, so the ores are oxides ...
Mineral
... KINDS OF MINERALS The 20 most common minerals are called rock-forming minerals because they form the rocks that make up Earth’s crust. Ten minerals are so common that they make up 90% of Earth’s crust. These minerals are quartz, orthoclase, plagioclase, muscovite, biotite, calcite, dolomite, halite ...
... KINDS OF MINERALS The 20 most common minerals are called rock-forming minerals because they form the rocks that make up Earth’s crust. Ten minerals are so common that they make up 90% of Earth’s crust. These minerals are quartz, orthoclase, plagioclase, muscovite, biotite, calcite, dolomite, halite ...
Mining
... • Public lands must be leased for mining, and royalties on profits are to be paid • Amounts depend on the resource being mined • Covers fossil fuels, sodium, sulfur, and phosphates ...
... • Public lands must be leased for mining, and royalties on profits are to be paid • Amounts depend on the resource being mined • Covers fossil fuels, sodium, sulfur, and phosphates ...
Lecture 32: Carbonates and Phosphates
... The frontiers of monazite research. Integrating accessory phase composition and geochronology into rock‐forming processes. Using combinations of common rock‐forming minerals (i.e. garnet‐biotite) Geologists can estimate the pressure and temperature at which mineral assemblages formed. If we can ...
... The frontiers of monazite research. Integrating accessory phase composition and geochronology into rock‐forming processes. Using combinations of common rock‐forming minerals (i.e. garnet‐biotite) Geologists can estimate the pressure and temperature at which mineral assemblages formed. If we can ...
I Struck Gold(?) at the Library
... it look like gold. In fact, pyrite is known as “fool’s gold.” Pyrite and gold are both minerals. Minerals are naturally occurring inorganic (not living) solids that have a distinctive chemical structure and are characterized by distinctive physical properties such as crystal structure, hardness, lus ...
... it look like gold. In fact, pyrite is known as “fool’s gold.” Pyrite and gold are both minerals. Minerals are naturally occurring inorganic (not living) solids that have a distinctive chemical structure and are characterized by distinctive physical properties such as crystal structure, hardness, lus ...
Minerals and Rocks
... 3. Some minerals form when magma and lava cools. Size of crystals depends upon: -- amount of gas the magma contains, -- chemical composition -- rate at which it cools: magma cools slowly so large crystals form lava cools quickly creating small crystals. Magma and lava contains oxygen and silico ...
... 3. Some minerals form when magma and lava cools. Size of crystals depends upon: -- amount of gas the magma contains, -- chemical composition -- rate at which it cools: magma cools slowly so large crystals form lava cools quickly creating small crystals. Magma and lava contains oxygen and silico ...
MINERALS: the building blocks of rocks There are five principal
... Ionic bonds occur as the result of the attractive force between atoms with opposite electrical charge, i.e. ‘opposites attract’. They occur between ions, which are stable charged forms of atoms. Ions form because of the tendency of atoms to obey the ‘rule of octet’, which states that atoms are more ...
... Ionic bonds occur as the result of the attractive force between atoms with opposite electrical charge, i.e. ‘opposites attract’. They occur between ions, which are stable charged forms of atoms. Ions form because of the tendency of atoms to obey the ‘rule of octet’, which states that atoms are more ...
BAG 101:
... A crystal face is a growth surface and a cleavage face is a breakage surface. Lustre: the quality and intensity of the light reflected from the mineral. Colour: often a striking property but not a very reliable means of identification. Streak: can be misleading, but often used. Hardness: refers to t ...
... A crystal face is a growth surface and a cleavage face is a breakage surface. Lustre: the quality and intensity of the light reflected from the mineral. Colour: often a striking property but not a very reliable means of identification. Streak: can be misleading, but often used. Hardness: refers to t ...
Normal Matter is composed of…….
... • Ex.) in a molecule of water - two atoms of hydrogen and one atom of oxygen share electrons. H2O ...
... • Ex.) in a molecule of water - two atoms of hydrogen and one atom of oxygen share electrons. H2O ...
earth-1st-edition-thompson-test-bank
... 12. Ninety-two percent of the Earth’s crust is composed of __________ minerals. 13. A/an __________ is a mineral that is prized for its rarity and beauty. 14. From __________ __________, metals or other elements can be recovered profitably. 15. A group of minerals that crystallize as long, thin fibe ...
... 12. Ninety-two percent of the Earth’s crust is composed of __________ minerals. 13. A/an __________ is a mineral that is prized for its rarity and beauty. 14. From __________ __________, metals or other elements can be recovered profitably. 15. A group of minerals that crystallize as long, thin fibe ...
4 - Earth materials
... – Protons (+), neutrons (0), and electrons (-) – Protons and neutrons hang out in the nucleus – Electrons (e-) reside in electron shells (orbitals), surrounding the nucleus Nucleus of atom = neutrons + protons: – neutrons mass of 1(amu) and charge of 0 – Protons mass of 1(amu) and charge ...
... – Protons (+), neutrons (0), and electrons (-) – Protons and neutrons hang out in the nucleus – Electrons (e-) reside in electron shells (orbitals), surrounding the nucleus Nucleus of atom = neutrons + protons: – neutrons mass of 1(amu) and charge of 0 – Protons mass of 1(amu) and charge ...
Physical properties of minerals
... composed of atoms, arranged in a specific order, with a well defined chemical composition. We might expect then that the microscopic variations in bond environment discussed above, will also be manifested in macroscopic physical and chemical properties. This is indeed the case. ...
... composed of atoms, arranged in a specific order, with a well defined chemical composition. We might expect then that the microscopic variations in bond environment discussed above, will also be manifested in macroscopic physical and chemical properties. This is indeed the case. ...
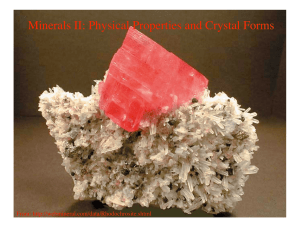
Minerals II: Physical Properties and Crystal Forms
... composed of atoms, arranged in a specific order, with a well defined chemical composition. We might expect then that the microscopic variations in bond environment discussed above, will also be manifested in macroscopic physical and chemical properties. This is indeed the case. ...
... composed of atoms, arranged in a specific order, with a well defined chemical composition. We might expect then that the microscopic variations in bond environment discussed above, will also be manifested in macroscopic physical and chemical properties. This is indeed the case. ...
Silicate Minerals
... A. Changing scales to looking at the elements of the earth and its crust (8 most common) B. Introduction to minerals that comprise rocks (11 most common minerals) C. The silicate minerals (7) D. Other important rock-forming minerals (4) E. Mineral properties ...
... A. Changing scales to looking at the elements of the earth and its crust (8 most common) B. Introduction to minerals that comprise rocks (11 most common minerals) C. The silicate minerals (7) D. Other important rock-forming minerals (4) E. Mineral properties ...
Rock Cycle - TeacherWeb
... The diagram in the next slide represents the ROCK CYCLE—a scheme that represents the processes of continuous changes that connect the three major groups of rocks: SEDIMENTARY IGNEOUS METAMORPHIC It also shows two other important parts of the “Rock Cycle” – SEDIMENTS and molten LAVA and MAGMA Note a ...
... The diagram in the next slide represents the ROCK CYCLE—a scheme that represents the processes of continuous changes that connect the three major groups of rocks: SEDIMENTARY IGNEOUS METAMORPHIC It also shows two other important parts of the “Rock Cycle” – SEDIMENTS and molten LAVA and MAGMA Note a ...
or here in RTF format
... variation, xenoliths, clasts, or other structures which may not be so obvious down the microscope. For many coarse-grained rocks, this is the easiest way to estimate average grain-size. (2) If large grains of opaque minerals are present, examine these in reflected light, using a hand lens if necessa ...
... variation, xenoliths, clasts, or other structures which may not be so obvious down the microscope. For many coarse-grained rocks, this is the easiest way to estimate average grain-size. (2) If large grains of opaque minerals are present, examine these in reflected light, using a hand lens if necessa ...
Conflict resource

Conflict resources are natural resources extracted in a conflict zone and sold to perpetuate the fighting. There is both anecdotal and statistical evidence that belligerent accessibility to precious commodities can prolong conflicts (a ""resource curse""). The most prominent contemporary example is the eastern provinces of the Democratic Republic of the Congo, where various armies, rebel groups, and outside actors have profited while contributing to violence and exploitation during wars in the region.The most commonly mined conflict minerals are cassiterite (for tin), wolframite (for tungsten), coltan (for tantalum), and gold ore, which are extracted from the Eastern Congo, and passed through a variety of intermediaries before being purchased by multinational electronics companies. These minerals are essential in the manufacture of a variety of devices, including consumer electronics such as mobile phones, laptops, and MP3 players.The extraction and sale of blood diamonds, also known as ""conflict diamonds"", is a better-known phenomenon which occurs under virtually identical conditions.Various international efforts have been made to reduce trade in conflict resources, to reduce the incentive to extract and fight over them. For example, in the United States, the 2010 Dodd–Frank Wall Street Reform and Consumer Protection Act requires manufacturers to audit their supply chains and report conflict minerals usage.