10.626 Lecture Notes, Electrochemical energy storage
... one, in the region 0 < x < L. The electrolyte-filled pore space has a constant volume-averaged resistance per length r and constant capacitance per unit length c. Neglect any resistance in the porous electrode or the thin gap between the electrodes. The mean potential in the pores satisfies a linear ...
... one, in the region 0 < x < L. The electrolyte-filled pore space has a constant volume-averaged resistance per length r and constant capacitance per unit length c. Neglect any resistance in the porous electrode or the thin gap between the electrodes. The mean potential in the pores satisfies a linear ...
Properties of Matter
... Law of conservation of matter : matter is neither created nor destroyed during a chemical reaction, ie., the total weight of substances before they react is the same as after they react. ...
... Law of conservation of matter : matter is neither created nor destroyed during a chemical reaction, ie., the total weight of substances before they react is the same as after they react. ...
Graphene Based Nanocomposite Electrodes for Energy storage in
... ABSTRACT Global warming and depleting fossil fuels have forced us to move towards sustainable and renewable resources for energy production. As the renewable resources of energy are not available on demand, energy storage systems are starting to play an important role in our lives. Currently the mos ...
... ABSTRACT Global warming and depleting fossil fuels have forced us to move towards sustainable and renewable resources for energy production. As the renewable resources of energy are not available on demand, energy storage systems are starting to play an important role in our lives. Currently the mos ...
Name: Date: Chapter 8-Lesson 3-5: Energy Transformations and
... temperaturea measure of the average energy of motion of the particles of a substance Fahrenheit scaleThe temperature scale on which water freezes at 32 degrees and boils at 212 degrees Celsius scaleThe temperature scale on which water freezes at 0 degrees and boils at 100 degrees Kelvin scaleThe tem ...
... temperaturea measure of the average energy of motion of the particles of a substance Fahrenheit scaleThe temperature scale on which water freezes at 32 degrees and boils at 212 degrees Celsius scaleThe temperature scale on which water freezes at 0 degrees and boils at 100 degrees Kelvin scaleThe tem ...
What is Energy?
... change in itself or the world around it. Whenever work is done, energy is transformed or is transferred from one system to another. ...
... change in itself or the world around it. Whenever work is done, energy is transformed or is transferred from one system to another. ...
Heat Energy - Waconia High School
... Example: Water (H2O) Breaking water into H & O will cause a release of chemical energy. ...
... Example: Water (H2O) Breaking water into H & O will cause a release of chemical energy. ...
Energy Vocabulary I
... thermal energy elastic energy insulation potential energy efficiency light gravitational potential energy conduction insulator electrical energy calories Law of Conservation of Energy energy transformation Fill in the blank with the correct term ...
... thermal energy elastic energy insulation potential energy efficiency light gravitational potential energy conduction insulator electrical energy calories Law of Conservation of Energy energy transformation Fill in the blank with the correct term ...
Energy Transformation Demos
... o Electrical Energy (energy of moving electrons) Mechanical energy is usually converted to electrical energy using a generator o Electromagnetic Energy Energy from Sun created by fusion…form ...
... o Electrical Energy (energy of moving electrons) Mechanical energy is usually converted to electrical energy using a generator o Electromagnetic Energy Energy from Sun created by fusion…form ...
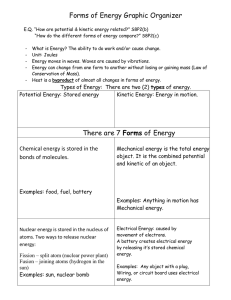
Forms of Energy
... What is Energy? The ability to do work and/or cause change. Unit: Joules Energy moves in waves. Waves are caused by vibrations. Energy can change from one form to another without losing or gaining mass (Law of Conservation of Mass). Heat is a byproduct of almost all changes in forms of energy. ...
... What is Energy? The ability to do work and/or cause change. Unit: Joules Energy moves in waves. Waves are caused by vibrations. Energy can change from one form to another without losing or gaining mass (Law of Conservation of Mass). Heat is a byproduct of almost all changes in forms of energy. ...
Energy storage

Energy storage is accomplished by devices or physical media that store energy to perform useful processes at a later time. A device that stores energy is sometimes called an accumulator.Many forms of energy produce useful work, heating or cooling to meet societal needs. These energy forms include chemical energy, gravitational potential energy, electrical potential, electricity, temperature differences, latent heat, and kinetic energy. Energy storage involves converting energy from forms that are difficult to store (electricity, kinetic energy, etc.) to more conveniently or economically storable forms. Some technologies provide only short-term energy storage, and others can be very long-term such as power to gas using hydrogen or methane and the storage of heat or cold between opposing seasons in deep aquifers or bedrock. A wind-up clock stores potential energy (in this case mechanical, in the spring tension), a rechargeable battery stores readily convertible chemical energy to operate a mobile phone, and a hydroelectric dam stores energy in a reservoir as gravitational potential energy. Ice storage tanks store ice (thermal energy in the form of latent heat) at night to meet peak demand for cooling. Fossil fuels such as coal and gasoline store ancient energy derived from sunlight by organisms that later died, became buried and over time were then converted into these fuels. Even food (which is made by the same process as fossil fuels) is a form of energy stored in chemical form.