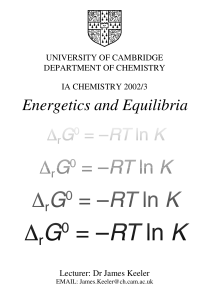Energy, Entropy and Exergy Concepts and Their Roles in Thermal
... • The microscopic forms of energy are those related to the molecular structure of a system and the degree of the molecular activity, and they are independent of outside reference frames. The sum of all the microscopic forms of energy is called the internal energy of a system. The internal energy of ...
... • The microscopic forms of energy are those related to the molecular structure of a system and the degree of the molecular activity, and they are independent of outside reference frames. The sum of all the microscopic forms of energy is called the internal energy of a system. The internal energy of ...
Thermodynamics Theory + Questions.0001
... Theory at a Glance (For GATE, IES & PSUs) Intensive and Extensive Properties Intensive property: Whose value is independent of the size or extent i.e. mass of the system. These are, e.g., pressure p and temperature T. Extensive property: Whose value depends on the size or extent i.e. mass of the sys ...
... Theory at a Glance (For GATE, IES & PSUs) Intensive and Extensive Properties Intensive property: Whose value is independent of the size or extent i.e. mass of the system. These are, e.g., pressure p and temperature T. Extensive property: Whose value depends on the size or extent i.e. mass of the sys ...
1 - Técnico Lisboa - Universidade de Lisboa
... Scalar theories try to describe the gravitational field using a scalar field, which must satisfy some field equation. Newton gravitation is a scalar field theory in the way that it is completely described by the potential φ which satisfies the Poisson equation. Indeed, the first attempts to create a ...
... Scalar theories try to describe the gravitational field using a scalar field, which must satisfy some field equation. Newton gravitation is a scalar field theory in the way that it is completely described by the potential φ which satisfies the Poisson equation. Indeed, the first attempts to create a ...
Microwave-Specific Effects on the Equilibrium Constants and
... ABSTRACT: The steam−carbon reaction, which is the essential reaction of the gasification processes of carbon-based feed stocks (e.g., coal and biomass), produces synthesis gas (H2 + CO), a synthetically flexible, environmentally benign energy source. The reaction is very endothermic, which mandates hi ...
... ABSTRACT: The steam−carbon reaction, which is the essential reaction of the gasification processes of carbon-based feed stocks (e.g., coal and biomass), produces synthesis gas (H2 + CO), a synthetically flexible, environmentally benign energy source. The reaction is very endothermic, which mandates hi ...
File - Association of Chemical Engineering Students
... The observations just described suggest a general restriction on processes beyond that imposed by the first law. The second law is equally well expressed in two statements that describe this restriction: ...
... The observations just described suggest a general restriction on processes beyond that imposed by the first law. The second law is equally well expressed in two statements that describe this restriction: ...
Document
... the species in a reaction and asked to determine in which direction the reaction must proceed to achieve equilibrium. Plan We can determine the starting concentration of each species in the reaction mixture. We can then substitute the starting concentrations into the equilibrium-constant expression ...
... the species in a reaction and asked to determine in which direction the reaction must proceed to achieve equilibrium. Plan We can determine the starting concentration of each species in the reaction mixture. We can then substitute the starting concentrations into the equilibrium-constant expression ...
Introduction
... these quantities are linked by equations, we are accustomed to detecting analogies between different physical theories through the similarity of the equations that describe their laws. Contrary to this practice, we have realized that to explain the origin of the analogies, we must not start from the ...
... these quantities are linked by equations, we are accustomed to detecting analogies between different physical theories through the similarity of the equations that describe their laws. Contrary to this practice, we have realized that to explain the origin of the analogies, we must not start from the ...
Entanglement Entropies in the Ground States of Helium
... values of Z . Our results for the linear entropy and the vN entropy are listed in the table 6, where a comparison with the literature [16,17] is also made. It is worth stressing that in each case considered here, the stability of the results up to at least six decimal places was achieved already at ...
... values of Z . Our results for the linear entropy and the vN entropy are listed in the table 6, where a comparison with the literature [16,17] is also made. It is worth stressing that in each case considered here, the stability of the results up to at least six decimal places was achieved already at ...
Energetics and Equilibria
... the surroundings from the heat change; here we will discuss more about how the entropy change of the system can be determined. The Second Law is easier to apply if we express it in terms of the Gibbs energy, G, and we saw that the Gibbs energy decreases in a spontaneous process. We will have more to ...
... the surroundings from the heat change; here we will discuss more about how the entropy change of the system can be determined. The Second Law is easier to apply if we express it in terms of the Gibbs energy, G, and we saw that the Gibbs energy decreases in a spontaneous process. We will have more to ...
Kinetics of vanadium carbonitride precipitation in steel: A computer
... According to our assumptions, Eq. (2) does not include the regular solution parameters that would otherwise account for interactions between VC and VN. In other words, we have neglected the excess molar free energy of mixing of VC and VN. Let us further assume that the austenite solid solution is di ...
... According to our assumptions, Eq. (2) does not include the regular solution parameters that would otherwise account for interactions between VC and VN. In other words, we have neglected the excess molar free energy of mixing of VC and VN. Let us further assume that the austenite solid solution is di ...
Selection of Thermodynamic Methods
... To establish if a real process is possible we need to consider: ∆G = ∆H − T ∆S The values for ∆H are determined from the heats of formation of the components and for ∆S from thermodynamic property tables. Superscript 0 indicates materials present in standard state at 298ºK. For isothermal processes ...
... To establish if a real process is possible we need to consider: ∆G = ∆H − T ∆S The values for ∆H are determined from the heats of formation of the components and for ∆S from thermodynamic property tables. Superscript 0 indicates materials present in standard state at 298ºK. For isothermal processes ...
Chapter 19 Thermodynamics - Farmingdale State College
... Thus, equation 19.11 represents the net work done by the gas in this particular cyclic process. Note that pA _ pD is one side of the rectangular path of figure 19.2(a) while VB _ VA is the other side of that rectangle. Hence, their product in equation 19.11 represents the entire area of the rectangl ...
... Thus, equation 19.11 represents the net work done by the gas in this particular cyclic process. Note that pA _ pD is one side of the rectangular path of figure 19.2(a) while VB _ VA is the other side of that rectangle. Hence, their product in equation 19.11 represents the entire area of the rectangl ...
Physical Science Degree
... A.apply basic concepts and fundamental laws in thermodynamics, electricity, and magnetism. B.solve problems in thermal expansion. C.differentiate the heat transfer mechanisms of conduction, convection, and radiation. D.apply the First Law of Thermodynamics. E.understand the relationship between temp ...
... A.apply basic concepts and fundamental laws in thermodynamics, electricity, and magnetism. B.solve problems in thermal expansion. C.differentiate the heat transfer mechanisms of conduction, convection, and radiation. D.apply the First Law of Thermodynamics. E.understand the relationship between temp ...
Exergy: the quality of energy
... Thermodynamics are based on experience, experience with nature that shows which conversions from one kind of energy into the other are possible and which are not. In the following several kinds of energy will play a role like: kinetic energy, potential energy, internal energy, heat, work, electrical ...
... Thermodynamics are based on experience, experience with nature that shows which conversions from one kind of energy into the other are possible and which are not. In the following several kinds of energy will play a role like: kinetic energy, potential energy, internal energy, heat, work, electrical ...
Thermodynamics of a pure substance at the triple point
... solid, liquid, and vapor phases coexist, but other triple points involving any three phases 共for example, polymorphic solids and 4He兲 can be formed. The essential feature of a triple point is that r = 0, that is, there are no independent intensive variables at the triple point. The pure substance is ...
... solid, liquid, and vapor phases coexist, but other triple points involving any three phases 共for example, polymorphic solids and 4He兲 can be formed. The essential feature of a triple point is that r = 0, that is, there are no independent intensive variables at the triple point. The pure substance is ...
Chemical Thermodynamics
... (For more information on matter, see Chapter 1 "Introduction to Chemistry".) The law of conservation of mass is the basis for all the stoichiometry and equilibrium calculations you have learned thus far in chemistry. The second, the law of conservation of energy, states that energy can be neither cr ...
... (For more information on matter, see Chapter 1 "Introduction to Chemistry".) The law of conservation of mass is the basis for all the stoichiometry and equilibrium calculations you have learned thus far in chemistry. The second, the law of conservation of energy, states that energy can be neither cr ...