Friction force: from mechanics to thermodynamics
... in the presence of friction taking into account the thermodynamical aspects, since it is well known that the temperature increases as soon as friction is introduced. Previous analysis in similar directions have been done4–8 . They differ from our approach in which we use observables defined at each ...
... in the presence of friction taking into account the thermodynamical aspects, since it is well known that the temperature increases as soon as friction is introduced. Previous analysis in similar directions have been done4–8 . They differ from our approach in which we use observables defined at each ...
Chapter 5 auxiliary functions
... * starting with system occurring in the first of these two states , ί . e ,the solid crystalline state ; the atom in such case are held together by interatomic force ; thus , if an atom to be removed from the crystal surface and placed in vapor phase ( the first atom is placed in vacuum) , energy i ...
... * starting with system occurring in the first of these two states , ί . e ,the solid crystalline state ; the atom in such case are held together by interatomic force ; thus , if an atom to be removed from the crystal surface and placed in vapor phase ( the first atom is placed in vacuum) , energy i ...
Introduction to the second law
... placed in thermal contact. The power of the second law is that it can be used to determine the equlibrium conditions for any thermodynamic system, under arbitrary constraints – e.g. systems that can exchange heat, exchange molecules, exchange electric charge, etc.- in response to arbitrary thermodyn ...
... placed in thermal contact. The power of the second law is that it can be used to determine the equlibrium conditions for any thermodynamic system, under arbitrary constraints – e.g. systems that can exchange heat, exchange molecules, exchange electric charge, etc.- in response to arbitrary thermodyn ...
Entropy, Carnot Engine and Thermoelectric Effect
... reverse the direction of flow of current, heat is still produced in the resistance but it cannot be used to convert heat into electrical energy. However, there is an another process by which heat can be converted into electrical energy and this phenomenon is called THERMOELECTRIC EFFECT. ...
... reverse the direction of flow of current, heat is still produced in the resistance but it cannot be used to convert heat into electrical energy. However, there is an another process by which heat can be converted into electrical energy and this phenomenon is called THERMOELECTRIC EFFECT. ...
full paper PDF format
... energy flows through industrial systems. The global industrial economy can be modeled as a network of industrial processes that extract resources from the Earth and transform those resources into commodities which can be bought and sold to meet the needs of humanity. Industrial ecology seeks to quan ...
... energy flows through industrial systems. The global industrial economy can be modeled as a network of industrial processes that extract resources from the Earth and transform those resources into commodities which can be bought and sold to meet the needs of humanity. Industrial ecology seeks to quan ...
History of Thermodynamics
... 1.3 Philosophy of science note As with science in general, thermodynamics is based on empirical observation. Moreover, it is important that those observations be repeatable. A few postulates, also known as axioms, will serve as the foundation of our science. Following Occam’s razor,9 we shall seek a ...
... 1.3 Philosophy of science note As with science in general, thermodynamics is based on empirical observation. Moreover, it is important that those observations be repeatable. A few postulates, also known as axioms, will serve as the foundation of our science. Following Occam’s razor,9 we shall seek a ...
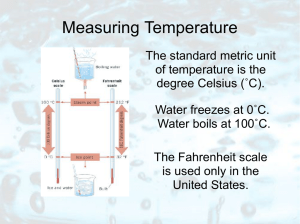
Measuring Temperature
... stated as the Law of Entropy: The total entropy (or microscopic disorganization) of the participants in any physical process cannot decrease during that process, but it can increase. That's not to say that you can't create a little more order in some part of your world, but the price you pay is to c ...
... stated as the Law of Entropy: The total entropy (or microscopic disorganization) of the participants in any physical process cannot decrease during that process, but it can increase. That's not to say that you can't create a little more order in some part of your world, but the price you pay is to c ...
Chapter 1 Classical Thermodynamics: The First Law 1.1 Introduction
... the change. Clearly, heat flow is only reversible if the temperature difference between the bodies is infinitesimally small. For a process to be reversible two conditions must be satisfied: (i) it must be quasistatic, and (ii) there must be no friction or other hysteresis effects present. A quasista ...
... the change. Clearly, heat flow is only reversible if the temperature difference between the bodies is infinitesimally small. For a process to be reversible two conditions must be satisfied: (i) it must be quasistatic, and (ii) there must be no friction or other hysteresis effects present. A quasista ...
Notes on the First Law of Thermodynamics Chemistry CHEM 213W
... Suppose this were not the case. Then you could presumably find a process which produced more work than it absorbed heat. This extra work could be used to run a generator, which in turn could be used to produce more heat, which could run more of process, producing even more excess work, and so on. Th ...
... Suppose this were not the case. Then you could presumably find a process which produced more work than it absorbed heat. This extra work could be used to run a generator, which in turn could be used to produce more heat, which could run more of process, producing even more excess work, and so on. Th ...
Entropy, a statistical approach
... o The change in entropy of a system depends only on the initial and final states – not on the path taken from one to the other. This is true for any state function. o If a system has possible microstates then doubling the size of the system will double the entropy (by increasing the number of poss ...
... o The change in entropy of a system depends only on the initial and final states – not on the path taken from one to the other. This is true for any state function. o If a system has possible microstates then doubling the size of the system will double the entropy (by increasing the number of poss ...
Chapter 17 notes ppt
... (randomness) = increasing in entropy • Third Law = entropy of a perfect crystal is zero at 0K = (absolute entropy can be determined for any temp higher than 0K) ...
... (randomness) = increasing in entropy • Third Law = entropy of a perfect crystal is zero at 0K = (absolute entropy can be determined for any temp higher than 0K) ...
Entropy and Free Energy
... ways could we distribute them among the volume elements so that white is on one side and black is on the other? On the other hand, how many ways could we distribute them with no restriction on which goes where? Clearly, there are many more ways to arrange them in the latter case. We measure "disorde ...
... ways could we distribute them among the volume elements so that white is on one side and black is on the other? On the other hand, how many ways could we distribute them with no restriction on which goes where? Clearly, there are many more ways to arrange them in the latter case. We measure "disorde ...
Thermodynamics - StrikerPhysics
... • We use macroscopic means for analysis of these systems of many particles - involving quantities such as pressure, volume and temperature. ...
... • We use macroscopic means for analysis of these systems of many particles - involving quantities such as pressure, volume and temperature. ...
Course Overview - Colorado State University College of Engineering
... polytropic exponent was measured to be 1.175. Based on the temperatures and pressures at the inlet and exit, the specific internal energy, u, at each state is known to be 234.9 kJ/kg to 267.5 kJ/kg, respectively. Also, based on the pressure, temperature and volume at state 1, it is known that each c ...
... polytropic exponent was measured to be 1.175. Based on the temperatures and pressures at the inlet and exit, the specific internal energy, u, at each state is known to be 234.9 kJ/kg to 267.5 kJ/kg, respectively. Also, based on the pressure, temperature and volume at state 1, it is known that each c ...