EXAM # 1
... the molecular formula of an unknown? AES can monitor the presence of up to 55 elements in an unknown sample by the observation of the presence/absence of the unique pattern of atomic emission bands for each element. Since AES follows Beer’s law, the relative intensity of the bands indicate the relat ...
... the molecular formula of an unknown? AES can monitor the presence of up to 55 elements in an unknown sample by the observation of the presence/absence of the unique pattern of atomic emission bands for each element. Since AES follows Beer’s law, the relative intensity of the bands indicate the relat ...
8.5DF: Chemical Formulas and Equations
... while cooking. Interestingly, there are many different ways that chemical reactions and chemical equations are used in cooking. For example, when you bake a cake, one of the chemical reactions that occurs is the baking soda reacting with water to produce carbon dioxide gas. This gas produces the “ho ...
... while cooking. Interestingly, there are many different ways that chemical reactions and chemical equations are used in cooking. For example, when you bake a cake, one of the chemical reactions that occurs is the baking soda reacting with water to produce carbon dioxide gas. This gas produces the “ho ...
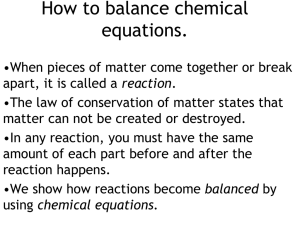
How to balance chemical equations.
... How to balance chemical equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coeffici ...
... How to balance chemical equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coeffici ...
Importance of Molecular Simulation for Studying Structural Properties
... A wide range of model energies are in use, depending on the type of system and the desired level of understanding. To obtain quantitatively accurate and chemically specific predictions, one uses ab initio energy surfaces, that is to say surfaces obtained from a quantum mechanical model for the syste ...
... A wide range of model energies are in use, depending on the type of system and the desired level of understanding. To obtain quantitatively accurate and chemically specific predictions, one uses ab initio energy surfaces, that is to say surfaces obtained from a quantum mechanical model for the syste ...
Chemical Properties - Michigan State University
... substances, which have different properties, compared to the old substances. The composition is a chemical change is altered. This occurs when you burn a substance, mixing an acid and a base, or when you observe rusting or rotting. The process that produces a chemical change is known as a chemical ...
... substances, which have different properties, compared to the old substances. The composition is a chemical change is altered. This occurs when you burn a substance, mixing an acid and a base, or when you observe rusting or rotting. The process that produces a chemical change is known as a chemical ...
МІНІСТЕРСТВО ОХОРОНИ ЗДОРОВ`Я УКРАЇНИ ХАРКІВСЬКИЙ
... algebraic sum of the enthalpies of formation of the reaction products less the algebraic sum of the enthalpies of formation of the reactants, with account of stoichiometric coefficients (first corollary). The second corollary of Hess’s law relates to heats of combustion (oxidation with oxygen): heat ...
... algebraic sum of the enthalpies of formation of the reaction products less the algebraic sum of the enthalpies of formation of the reactants, with account of stoichiometric coefficients (first corollary). The second corollary of Hess’s law relates to heats of combustion (oxidation with oxygen): heat ...
H 2 O
... • Volume – Temperature Relationship – At constant pressure, the volume is directly proportional to temperature ...
... • Volume – Temperature Relationship – At constant pressure, the volume is directly proportional to temperature ...
about a variety of material equilibrium conditions
... In open systems entropy S and volume V changes with necessity at change of common number of moles of system N (by mass transfer), and also at change of its composition (by diffusion). That circumstance breaks a condition of its constancy, underlying in above mentioned definition of chemical potentia ...
... In open systems entropy S and volume V changes with necessity at change of common number of moles of system N (by mass transfer), and also at change of its composition (by diffusion). That circumstance breaks a condition of its constancy, underlying in above mentioned definition of chemical potentia ...
I Examen I Trim Science
... A solid is the state of matter that has a definite shape and volume. The particles in a solid do not move fast enough to overcome the attraction between them. Each particle vibrates in place and is locked in place by the particles around it. 2 types of solids: Crystalline solids: have a very ...
... A solid is the state of matter that has a definite shape and volume. The particles in a solid do not move fast enough to overcome the attraction between them. Each particle vibrates in place and is locked in place by the particles around it. 2 types of solids: Crystalline solids: have a very ...
CHM – 124 Principles of Chemistry
... Equivalent weights of acids and bases. Acid-base titration. Colligative properties of solutions. Section XIV - ...
... Equivalent weights of acids and bases. Acid-base titration. Colligative properties of solutions. Section XIV - ...
Monday, February 5, 2007
... • An electron placed at point b will move toward the positive plate since it was released at its highest potential energy point. • It will gain kinetic energy as it moves toward left, decreasing its potential energy. • The electron, however, moves from the point b at a lower potential to point a at ...
... • An electron placed at point b will move toward the positive plate since it was released at its highest potential energy point. • It will gain kinetic energy as it moves toward left, decreasing its potential energy. • The electron, however, moves from the point b at a lower potential to point a at ...
Chapter 9 Notes - Get a Clue with Mrs. Perdue
... In the chemical equation below, put a BOX around the product and CIRCLE the reactants ...
... In the chemical equation below, put a BOX around the product and CIRCLE the reactants ...
Handout - Notes - 4 - Electric Potential and Voltage
... object moving downward within the gravitational field would lose gravitational potential energy. When gravitational potential energy was introduced in, it was defined as the energy stored in an object due to its vertical position above the Earth. The amount of gravitational potential energy stored i ...
... object moving downward within the gravitational field would lose gravitational potential energy. When gravitational potential energy was introduced in, it was defined as the energy stored in an object due to its vertical position above the Earth. The amount of gravitational potential energy stored i ...
chemical reaction
... are never lost or gained in a chemical reaction, they are just rearranged. Every atom in the reactants becomes part of the products. • When writing a chemical equation, make sure the number of atoms of each element in the reactants equals the number of atoms of those same elements in the products. T ...
... are never lost or gained in a chemical reaction, they are just rearranged. Every atom in the reactants becomes part of the products. • When writing a chemical equation, make sure the number of atoms of each element in the reactants equals the number of atoms of those same elements in the products. T ...
UA-CHEM 127: Advanced General Chemistry I
... including metal oxides, carbonates and sulfides. From the work of Robert Boyle in the 17th century, it was understood that substances that could be broken down into more fundamental components were mixtures or compounds. Substances that could not be further broken down were referred to as elements. ...
... including metal oxides, carbonates and sulfides. From the work of Robert Boyle in the 17th century, it was understood that substances that could be broken down into more fundamental components were mixtures or compounds. Substances that could not be further broken down were referred to as elements. ...