File
... from one phase or state of matter to another one by heat transfer. The term is most commonly used to describe transitions between solid, liquid and gaseous states of matter, and, in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. Durin ...
... from one phase or state of matter to another one by heat transfer. The term is most commonly used to describe transitions between solid, liquid and gaseous states of matter, and, in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. Durin ...
Chapter 4
... needed to increase the temperature of 1 kg of water by 1oC. • In the English system the heat unit is the BTU (British thermal unit). This one is the amount of heat needed to increase the temperature on 1 pound of water by 1oF. ...
... needed to increase the temperature of 1 kg of water by 1oC. • In the English system the heat unit is the BTU (British thermal unit). This one is the amount of heat needed to increase the temperature on 1 pound of water by 1oF. ...
Thermodynamics
... expressed in terms of units or degrees designated on a standard scale". The most commonly used temperature scale is Celsius, which is based on the freezing and boiling points of water, assigning respective values of 0 degrees C and 100 degrees C. The Fahrenheit scale is also based on the freezing an ...
... expressed in terms of units or degrees designated on a standard scale". The most commonly used temperature scale is Celsius, which is based on the freezing and boiling points of water, assigning respective values of 0 degrees C and 100 degrees C. The Fahrenheit scale is also based on the freezing an ...
Molar Heat Capacities of an Ideal Gas
... density. It is replaced by cooler, denser liquid that then becomes heated and rises. Upon reaching to the top, it cools and hence sinks back to the bottom. These convection currents continue to flow through the liquid. A similar situation occurs on the outer surface of the sun. The granules observed ...
... density. It is replaced by cooler, denser liquid that then becomes heated and rises. Upon reaching to the top, it cools and hence sinks back to the bottom. These convection currents continue to flow through the liquid. A similar situation occurs on the outer surface of the sun. The granules observed ...
PPT version
... insulated from everything else). • A thermometer must be much smaller than system. • For fast temperature measurements, it should be small, have good thermal conductivity and low heat capacity. ...
... insulated from everything else). • A thermometer must be much smaller than system. • For fast temperature measurements, it should be small, have good thermal conductivity and low heat capacity. ...
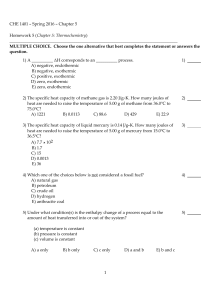
CHE 1401 - Summer 2012 - Chapter 5 Homework 5 (Chapter 5
... B) A negative ΔH corresponds to an exothermic process. C) ΔE = Efinal - Einitial D) Energy lost by the system must be gained by the surroundings. E) 1 cal = 4.184 J (exactly) 9) The British thermal unit (Btu) is commonly used in engineering applications. A Btu is defined as the amount of heat requir ...
... B) A negative ΔH corresponds to an exothermic process. C) ΔE = Efinal - Einitial D) Energy lost by the system must be gained by the surroundings. E) 1 cal = 4.184 J (exactly) 9) The British thermal unit (Btu) is commonly used in engineering applications. A Btu is defined as the amount of heat requir ...
CHE 1401 - Fall 2016 - Chapter 5 Homework 5 (Chapter 5
... is __________, and therefore heat is __________ by the reaction. A) exothermic, absorbed B) exothermic, released C) endothermic, released D) endothermic, absorbed E) thermoneutral, neither released nor absorbed 27) Which of the following is a statement of Hess's law? A) The ΔH for a process in the f ...
... is __________, and therefore heat is __________ by the reaction. A) exothermic, absorbed B) exothermic, released C) endothermic, released D) endothermic, absorbed E) thermoneutral, neither released nor absorbed 27) Which of the following is a statement of Hess's law? A) The ΔH for a process in the f ...
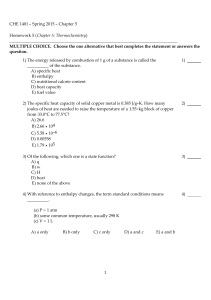
CHE 1401 - Spring 2015 - Chapter 5 Homework 5 (Chapter 5
... E) none of the above is always negative. ...
... E) none of the above is always negative. ...
Chapter 9: Thermodynamic Processes and Thermochemistry
... expressed the tendency of reactants to be converted to products in terms of the equilibrium constant K for the reaction. Now we question why some reactions have large equilibrium constants while other reactions have small equilibrium constants. The goal of chemical thermodynamics is to predict what ...
... expressed the tendency of reactants to be converted to products in terms of the equilibrium constant K for the reaction. Now we question why some reactions have large equilibrium constants while other reactions have small equilibrium constants. The goal of chemical thermodynamics is to predict what ...
Notes
... Hsolution , sometimes called heat of solution or molar enthalpy of dissolution, refers to the Hrxn for the physical process of dissolving one mole of a substance in water. ...
... Hsolution , sometimes called heat of solution or molar enthalpy of dissolution, refers to the Hrxn for the physical process of dissolving one mole of a substance in water. ...
PPT
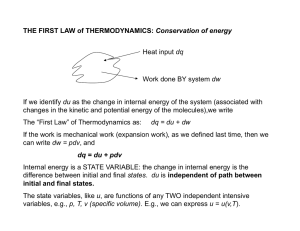
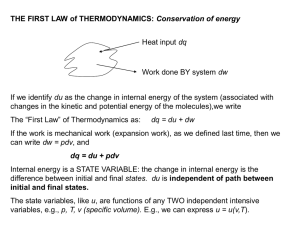
... To study this process further – consider the CARNOT CYCLE (a cyclic process) CYCLIC PROCESS – a series of change in the state of a substance in which its volume changes and it does work, with the substance returning to its original state. Initial & final state are identical in a cyclic process. Sinc ...
... To study this process further – consider the CARNOT CYCLE (a cyclic process) CYCLIC PROCESS – a series of change in the state of a substance in which its volume changes and it does work, with the substance returning to its original state. Initial & final state are identical in a cyclic process. Sinc ...