Questions - TTU Physics
... 2. Consider a classical plane pendulum. The mass m, is suspended from the ceiling by a massless wire of length ℓ. It oscillates in a plane with no friction. The total mechanical energy is E = [(L2)/(2mℓ2)] + mgℓ(1 – cosθ). L is the angular momentum about the suspension point. θ is the oscillation an ...
... 2. Consider a classical plane pendulum. The mass m, is suspended from the ceiling by a massless wire of length ℓ. It oscillates in a plane with no friction. The total mechanical energy is E = [(L2)/(2mℓ2)] + mgℓ(1 – cosθ). L is the angular momentum about the suspension point. θ is the oscillation an ...
The Laws of Thermodynamics
... immersed in a liquid. Once steady state is reached, the state of the coil does not change in any way, and all of the electrical energy goes into heating the liquid. Similarly, when mechanical work is done to overcome friction, it too can be done in such a way that all the mechanical energy is conver ...
... immersed in a liquid. Once steady state is reached, the state of the coil does not change in any way, and all of the electrical energy goes into heating the liquid. Similarly, when mechanical work is done to overcome friction, it too can be done in such a way that all the mechanical energy is conver ...
Unit 6 Review
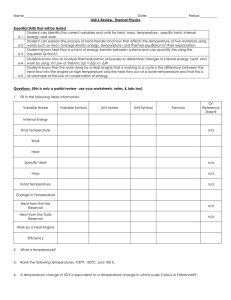
... Name __________________________________________________________________ Date: _______________________ Period __________ Unit 6 Review- Thermal Physics Essential Skills that will be tested Student can identify the correct variables and units for heat, mass, temperature, specific heat, internal 6-1 en ...
... Name __________________________________________________________________ Date: _______________________ Period __________ Unit 6 Review- Thermal Physics Essential Skills that will be tested Student can identify the correct variables and units for heat, mass, temperature, specific heat, internal 6-1 en ...
Thermodynamics
... thermodynamics the internal energy of a thermodynamic system, or a body with well-defined boundaries, denoted by U, or sometimes E, is the total of the kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the potential energy associated with the vibrational and ...
... thermodynamics the internal energy of a thermodynamic system, or a body with well-defined boundaries, denoted by U, or sometimes E, is the total of the kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the potential energy associated with the vibrational and ...
Quiz_MATH.rtf
... A certain humidifier operates by raising water to the boiling point and then evaporating it. Every minute 30 g of water at 20C are added to replace the 30 g that are evaporated. The heat of fusion of water is333 kJ/kg, the heat of vaporization is 2256 kj/kg, and the specific heat is 4190 J/kg ...
... A certain humidifier operates by raising water to the boiling point and then evaporating it. Every minute 30 g of water at 20C are added to replace the 30 g that are evaporated. The heat of fusion of water is333 kJ/kg, the heat of vaporization is 2256 kj/kg, and the specific heat is 4190 J/kg ...
Bagian 2 termodinamika
... Three different experiments are run, in which a gas expands from point A to point D along the three paths shown below. Calculate the amount of work done ...
... Three different experiments are run, in which a gas expands from point A to point D along the three paths shown below. Calculate the amount of work done ...
Calorimetry

Calorimetry is the science or act of measuring changes in state variables of a body for the purpose of deriving the heat transfer associated with changes of its state due for example to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat and the Greek word μέτρον (metron), meaning measure. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry.Indirect Calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The Dynamic Energy Budget theory explains why this procedure is correct. Heat generated by living organisms may also be measured by direct calorimetry, in which the entire organism is placed inside the calorimeter for the measurement.A widely used modern instrument is the differential scanning calorimeter, a device which allows thermal data to be obtained on small amounts of material. It involves heating the sample at a controlled rate and recording the heat flow either into or from the specimen.