chapter 4 crossword pre-ap
... 1. The electronegativity ___ as you move down a group on the periodic table. 3. Alkaline-earth metals have __ valence electrons. 4. The atomic radii of atoms ___ as you move left to right across a period. 5. Group 2 elements. 10. The number of valence electrons of a noble gas. 15. A reaction that af ...
... 1. The electronegativity ___ as you move down a group on the periodic table. 3. Alkaline-earth metals have __ valence electrons. 4. The atomic radii of atoms ___ as you move left to right across a period. 5. Group 2 elements. 10. The number of valence electrons of a noble gas. 15. A reaction that af ...
THE PERIODIC TABLE ChapterTestA
... 12. WhIch sublevel corresponds to the transition metals in the periodic table? c.d a.s d.f b.p 13. The representative elements are a. inner transition metals. b. transition metals. ...
... 12. WhIch sublevel corresponds to the transition metals in the periodic table? c.d a.s d.f b.p 13. The representative elements are a. inner transition metals. b. transition metals. ...
Physical Science Chapters 4
... Physical Science Chapters 4-5 Study Guide 1. Know all vocabulary from chapters 4-5. ...
... Physical Science Chapters 4-5 Study Guide 1. Know all vocabulary from chapters 4-5. ...
Subatomic Particles
... • Atoms of the same _________ that have the same number of ________ (p+) but different numbers of ________ (n°) are known as _________ of that element. • _________ of an element are represented b adding the number that indicates the ___________ (A) of hat isotope to the ...
... • Atoms of the same _________ that have the same number of ________ (p+) but different numbers of ________ (n°) are known as _________ of that element. • _________ of an element are represented b adding the number that indicates the ___________ (A) of hat isotope to the ...
Name: Per: _____ Date: ______ Unit 5 Redemption Packet: The
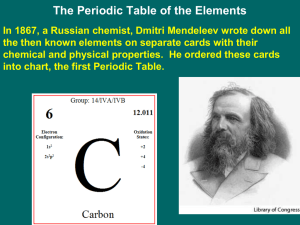
... questions. If you missed any notes, you will find them at www.starkchemistry.weebly.com. 1. Mendeleev arranged the elements in his periodic table by increasing _______________ ________________. 2. Why did Mendeleev leave spaces in his periodic table? _________________________________________________ ...
... questions. If you missed any notes, you will find them at www.starkchemistry.weebly.com. 1. Mendeleev arranged the elements in his periodic table by increasing _______________ ________________. 2. Why did Mendeleev leave spaces in his periodic table? _________________________________________________ ...
2.2 Periodic Chart
... The Atomic Mass Unit (amu) One atomic mass unit is about the weight of one proton. Exactly it is 1.66 x 10-27 kg. The Atomic Mass of each element in the Modern Periodic Chart is an average of its various isotopes (forms of the same element with different numbers of neutrons) in amus. ...
... The Atomic Mass Unit (amu) One atomic mass unit is about the weight of one proton. Exactly it is 1.66 x 10-27 kg. The Atomic Mass of each element in the Modern Periodic Chart is an average of its various isotopes (forms of the same element with different numbers of neutrons) in amus. ...
ATOMS ELEMENTS PERIODIC TABLE MOLECULES COMPOUNDS
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
Chapter 7:
... In the universe, there are 92 naturally occurring elements. The other elements are synthetic, or created in a laboratory. Remember, that elements in Groups 1A-8A are also called the representative elements. Remember that the number that comes in front of the A, tells how many valence electrons the e ...
... In the universe, there are 92 naturally occurring elements. The other elements are synthetic, or created in a laboratory. Remember, that elements in Groups 1A-8A are also called the representative elements. Remember that the number that comes in front of the A, tells how many valence electrons the e ...
The Periodic Table of Elements - PAMS-Doyle
... • These have properties that are similar to one another • All have 2 valence electrons • They have been moved to the bottom to make the ...
... • These have properties that are similar to one another • All have 2 valence electrons • They have been moved to the bottom to make the ...
Chapter6
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
Pre-class Activity 12/18
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
Chapter 5 student
... of elements in those groups. • Predict the reactivity of some elements based on their locations within a group. • Identify some properties of common A group elements. ...
... of elements in those groups. • Predict the reactivity of some elements based on their locations within a group. • Identify some properties of common A group elements. ...
Chapter 5
... • Left blank spots in table which predicted properties of elements not yet discovered ...
... • Left blank spots in table which predicted properties of elements not yet discovered ...
2.5-The Periodic Table
... take its place. He predicted the properties of the “unknown” elements, and within the next sixteen years those gaps were filled in with newly discovered elements that matched precisely with Mendeleev’s predictions! ...
... take its place. He predicted the properties of the “unknown” elements, and within the next sixteen years those gaps were filled in with newly discovered elements that matched precisely with Mendeleev’s predictions! ...
Periodic Table Cloze - Science
... _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the Calcium: an element on the periodic table with atomic number 20. ...
... _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the Calcium: an element on the periodic table with atomic number 20. ...
Chapter 5
... • The elements in Group_______ are called the noble gases • Helium has _______ valence electrons • All other noble gases have ______ valence electrons • The noble gases are________ and________ and extremely_________ • All the noble gases except ______ are used in “neon” lights ...
... • The elements in Group_______ are called the noble gases • Helium has _______ valence electrons • All other noble gases have ______ valence electrons • The noble gases are________ and________ and extremely_________ • All the noble gases except ______ are used in “neon” lights ...
Name - TeacherWeb
... 10. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth ...
... 10. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth ...
Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
Periodic Table and Elements Review
... b.) What is formed from an alloy of copper and zinc? 27.) Economically, what is the most important element of the Boron group, and what are some major benefits of this element? ...
... b.) What is formed from an alloy of copper and zinc? 27.) Economically, what is the most important element of the Boron group, and what are some major benefits of this element? ...
Date Period - Swift Classroom
... He put the elements in order by _______________________ He found that other properties such as _________________________, _______________________, and the ability to ______________________ with other elements seemed to ____________________ over and over. This repeating pattern is called ______ ...
... He put the elements in order by _______________________ He found that other properties such as _________________________, _______________________, and the ability to ______________________ with other elements seemed to ____________________ over and over. This repeating pattern is called ______ ...
ELEMENTS and THEIR PROPERTIES
... • He put the elements in order by their atomic mass, today we have them in order of their atomic number. • He left blank spaces in his table to keep the elements lined properly. ...
... • He put the elements in order by their atomic mass, today we have them in order of their atomic number. • He left blank spaces in his table to keep the elements lined properly. ...
Periodic table
... links. Below are some suggested sentences that relate to each of the numbers on the diagram. They are not the only sentences that are valid. ...
... links. Below are some suggested sentences that relate to each of the numbers on the diagram. They are not the only sentences that are valid. ...
The Modern Periodic Table
... d. Variation across a Period Across a period from left to right, the elements become less metallic and more nonmetallic in their properties. ...
... d. Variation across a Period Across a period from left to right, the elements become less metallic and more nonmetallic in their properties. ...
The Periodic Table - Harlan Independent Schools
... Charge is usually 2 but can vary— usually 2 valence electrons These are the metals you are probably most familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
... Charge is usually 2 but can vary— usually 2 valence electrons These are the metals you are probably most familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...