CHAPTER 1, MATTER AND CHANGE Section 1, Chemistry Is a
... stable substances and is made of one type of atom. (Example: hydrogen) ! A compound is a substance that can be broken down into simple stable substances. Each compound is made from the atoms of two or more elements that are chemically bonded. (Example: hydrogen peroxide, H2O2) Properties and changes ...
... stable substances and is made of one type of atom. (Example: hydrogen) ! A compound is a substance that can be broken down into simple stable substances. Each compound is made from the atoms of two or more elements that are chemically bonded. (Example: hydrogen peroxide, H2O2) Properties and changes ...
Intro to Physics - hrsbstaff.ednet.ns.ca
... Physics: Important Unit Terms Position = describes an object’s location. Time = describes when an event occurs. Distance = the total length of a journey along every twist and turn of the path. Displacement = describes how much an object’s ...
... Physics: Important Unit Terms Position = describes an object’s location. Time = describes when an event occurs. Distance = the total length of a journey along every twist and turn of the path. Displacement = describes how much an object’s ...
Elements and Atoms
... must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
... must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
Study Guide Answers
... protons? Why or Why not? Yes, every atom of the same element has to have the same number of protons. The number of protons determines the type of atom. Example, all hydrogen atoms have 1 proton and all helium atoms have 2 protons. 18. Which element is the only metal that is not a solid at room ...
... protons? Why or Why not? Yes, every atom of the same element has to have the same number of protons. The number of protons determines the type of atom. Example, all hydrogen atoms have 1 proton and all helium atoms have 2 protons. 18. Which element is the only metal that is not a solid at room ...
is the accelerating voltage of 1000 V)
... into a region of perpendicular electric and magnetic fields. If the electric field is generated by two parallel plates separated by 2.0 cm, what would the voltage between the two plates have to be so that the alpha particle passes through undeflected in a magnetic field of 0.92T? ; need to find ...
... into a region of perpendicular electric and magnetic fields. If the electric field is generated by two parallel plates separated by 2.0 cm, what would the voltage between the two plates have to be so that the alpha particle passes through undeflected in a magnetic field of 0.92T? ; need to find ...
Fermionic Vortices Find their Dual - Physics (APS)
... half-filled Landau level [7], the best-understood non-Fermi liquid state. Briefly, the successful theory of this state makes a distinction between half-filled and half-empty electronic bands. The associated particle-hole symmetry is emergent, and an exact local realization can only occur at the edge ...
... half-filled Landau level [7], the best-understood non-Fermi liquid state. Briefly, the successful theory of this state makes a distinction between half-filled and half-empty electronic bands. The associated particle-hole symmetry is emergent, and an exact local realization can only occur at the edge ...
PHYSICAL PROPERTIES OF SULFIDE MATERIALS
... the validity of the atomic energy levels calculated for the free atom. One of the basic concepts here is the idea of exchange interactions and covalent bonds. The central concept of the covalent bond is that a lower energy can be achieved by sharing electrons between neighboring atoms in directional ...
... the validity of the atomic energy levels calculated for the free atom. One of the basic concepts here is the idea of exchange interactions and covalent bonds. The central concept of the covalent bond is that a lower energy can be achieved by sharing electrons between neighboring atoms in directional ...
RIGHT-HAND RULE
... occurs. A list of physical quantities whose directions are related by the right-hand rule is given below. The angular velocity of a rotating object and the rotational velocity of any point on the object A torque, the force that causes it, and the position of the point of application of the force ...
... occurs. A list of physical quantities whose directions are related by the right-hand rule is given below. The angular velocity of a rotating object and the rotational velocity of any point on the object A torque, the force that causes it, and the position of the point of application of the force ...
Features of spin-orbit-induced dynamics in magnetic nanofilms
... possessing by the property of the field and current-govern magnetic dynamics with ultimately small energy consumption as base elements for nanodevices of an information technology with high bit densities and high-frequency radiation is related to the spin-orbit-induced torque exerting on magnetic st ...
... possessing by the property of the field and current-govern magnetic dynamics with ultimately small energy consumption as base elements for nanodevices of an information technology with high bit densities and high-frequency radiation is related to the spin-orbit-induced torque exerting on magnetic st ...
763645S SUPERCONDUCTIVITY Solutions 3 Fall 2015 1. Derive
... Note 2: This at least “justifies” the appearance of the “V H · dB” term in the differential for internal energy (dE) and thus the Helmholtz free energy (dF ), when we assume that ∂D and the “free currents” jf vanish. Existence of screening supercurrents induced by the ∂t applied magnetic field are s ...
... Note 2: This at least “justifies” the appearance of the “V H · dB” term in the differential for internal energy (dE) and thus the Helmholtz free energy (dF ), when we assume that ∂D and the “free currents” jf vanish. Existence of screening supercurrents induced by the ∂t applied magnetic field are s ...
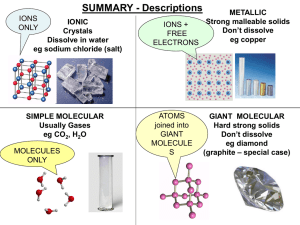
smart_materials_1 - Aldercar High School
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
Cathode Ray Tubes and The JJ Thompson Experiment
... Famed physicist J.J. Thompson took the cathode ray a step further. First he set up a cathode ray tube that deflected the electron ray using a second set of electrically charged plates (aka yoke), similar to the ...
... Famed physicist J.J. Thompson took the cathode ray a step further. First he set up a cathode ray tube that deflected the electron ray using a second set of electrically charged plates (aka yoke), similar to the ...
Introduction to Quantum Mechanics Homework #3 (Due on April 28
... on the wavefunction given in the textbook. Also calculate the numerical value of E0 in eV unit. 4. 1) What is the virial theorem? 2)Calculate the average potential energy of H1s electron in eV unit using the wavefunction given in the textbook. What is the average kinetic energy of the H1s electron? ...
... on the wavefunction given in the textbook. Also calculate the numerical value of E0 in eV unit. 4. 1) What is the virial theorem? 2)Calculate the average potential energy of H1s electron in eV unit using the wavefunction given in the textbook. What is the average kinetic energy of the H1s electron? ...
- NUS Physics
... SQUID is loop of superconductor that contains one or more Josephson Junctions. (interface between two superconducting materials separated by a non-superconducting barrier. A current may flow freely within the superconductors, but the barrier prevents the current from flowing freely between them. How ...
... SQUID is loop of superconductor that contains one or more Josephson Junctions. (interface between two superconducting materials separated by a non-superconducting barrier. A current may flow freely within the superconductors, but the barrier prevents the current from flowing freely between them. How ...
Midterm Review Sample Content Questions
... 9. Which of the following signs of a chemical reaction could potentially occur in a physical change too: color change, temperature change, gas evolution, formation of a precipitate, new substance forms. List any/all that apply and what conditions must occur for them to be considered physical by prov ...
... 9. Which of the following signs of a chemical reaction could potentially occur in a physical change too: color change, temperature change, gas evolution, formation of a precipitate, new substance forms. List any/all that apply and what conditions must occur for them to be considered physical by prov ...
Condensed matter physics

Condensed matter physics is a branch of physics that deals with the physical properties of condensed phases of matter. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, these include the laws of quantum mechanics, electromagnetism and statistical mechanics.The most familiar condensed phases are solids and liquids, while more exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on atomic lattices, and the Bose–Einstein condensate found in cold atomic systems. The study of condensed matter physics involves measuring various material properties via experimental probes along with using techniques of theoretical physics to develop mathematical models that help in understanding physical behavior.The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists identify themselves as condensed matter physicists, and the Division of Condensed Matter Physics is the largest division at the American Physical Society. The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics. Theoretical condensed matter physics shares important concepts and techniques with theoretical particle and nuclear physics.A variety of topics in physics such as crystallography, metallurgy, elasticity, magnetism, etc., were treated as distinct areas, until the 1940s when they were grouped together as solid state physics. Around the 1960s, the study of physical properties of liquids was added to this list, forming the basis for the new, related specialty of condensed matter physics. According to physicist Phil Anderson, the term was coined by him and Volker Heine when they changed the name of their group at the Cavendish Laboratories, Cambridge from ""Solid state theory"" to ""Theory of Condensed Matter"" in 1967, as they felt it did not exclude their interests in the study of liquids, nuclear matter and so on. Although Anderson and Heine helped popularize the name ""condensed matter"", it had been present in Europe for some years, most prominently in the form of a journal published in English, French, and German by Springer-Verlag titled Physics of Condensed Matter, which was launched in 1963. The funding environment and Cold War politics of the 1960s and 1970s were also factors that lead some physicists to prefer the name ""condensed matter physics"", which emphasized the commonality of scientific problems encountered by physicists working on solids, liquids, plasmas, and other complex matter, over ""solid state physics"", which was often associated with the industrial applications of metals and semiconductors. The Bell Telephone Laboratories was one of the first institutes to conduct a research program in condensed matter physics.References to ""condensed"" state can be traced to earlier sources. For example, in the introduction to his 1947 ""Kinetic theory of liquids"" book, Yakov Frenkel proposed that ""The kinetic theory of liquids must accordingly be developed as a generalization and extension of the kinetic theory of solid bodies"". As a matter of fact, it would be more correct to unify them under the title of ""condensed bodies"".