Chap 7: Around the Room Review
... 9. Who developed the first periodic table of the elements? 10. The elements in Group 1 are commonly called the _______. 11. The isotope nitrogen-13 has a half-life of 10 minutes. If you start with 40 grams, how many grams of this isotope will you have remaining after 30 minutes? Show your work. 12. ...
... 9. Who developed the first periodic table of the elements? 10. The elements in Group 1 are commonly called the _______. 11. The isotope nitrogen-13 has a half-life of 10 minutes. If you start with 40 grams, how many grams of this isotope will you have remaining after 30 minutes? Show your work. 12. ...
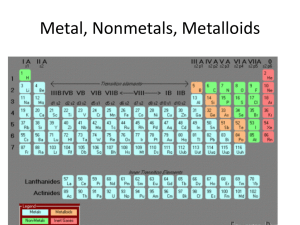
Ions and isotopes
... • the # of electrons and neutrons can change in an element without changing the identity of the element. ...
... • the # of electrons and neutrons can change in an element without changing the identity of the element. ...
Unit 2 Notes - School City of Hobart
... • For a nuclide to be considered radioactive, it must have an unstable nucleus due to an imbalance between # of protons and neutrons ~1:1 ratio = stable *The further an isotope is from a 1:1 ratio, the more likely it is to be radioactive (Figure 21.2, p.881) • Types of Nuclear Decay (aka Radioactivi ...
... • For a nuclide to be considered radioactive, it must have an unstable nucleus due to an imbalance between # of protons and neutrons ~1:1 ratio = stable *The further an isotope is from a 1:1 ratio, the more likely it is to be radioactive (Figure 21.2, p.881) • Types of Nuclear Decay (aka Radioactivi ...
Chapter 14 Review
... 16. The half-life of Nobelium is 3 minutes. How much Nobelium will there be in 12 minutes if the original sample was 48 grams? What has happened to the other part of the sample? ...
... 16. The half-life of Nobelium is 3 minutes. How much Nobelium will there be in 12 minutes if the original sample was 48 grams? What has happened to the other part of the sample? ...
Chapter 21 Powerpoint: Nuclear Chemistry
... How many half lives have passed if there is only 1.875 g left of a 30 g sample? ...
... How many half lives have passed if there is only 1.875 g left of a 30 g sample? ...
Chemical Basis of Life
... Title: The Chemical Basis of Life 1- Introduction: Your body is an elaborate chemical system. Chemical reactions power all of the body’s activities. At the most basic level, life is about chemicals and how they interact with each other. 2- Matter – Matter is anything that has mass and occupies space ...
... Title: The Chemical Basis of Life 1- Introduction: Your body is an elaborate chemical system. Chemical reactions power all of the body’s activities. At the most basic level, life is about chemicals and how they interact with each other. 2- Matter – Matter is anything that has mass and occupies space ...
Neptunium
.png?width=300)
Neptunium is a chemical element with symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is radioactive, pyrophoric, and can accumulate in bones, which makes the handling of neptunium dangerous.Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then, most neptunium has been and still is produced by neutron irradiation of uranium in nuclear reactors. The vast majority is generated as a by-product in conventional nuclear power reactors. While neptunium itself has no commercial uses at present, it is widely used as a precursor for the formation of plutonium-238, used in radioisotope thermal generators. Neptunium has also been used in detectors of high-energy neutrons.The most stable isotope of neptunium, neptunium-237, is a by-product of nuclear reactors and plutonium production. It, and the isotope neptunium-239, are also found in trace amounts in uranium ores due to neutron capture reactions and beta decay.