Atoms and the Periodic Table
... The elements are arranged on the periodic table by the number of protons and then grouped by other properties, such as: ...
... The elements are arranged on the periodic table by the number of protons and then grouped by other properties, such as: ...
PHYSICS E09 11
... 1. The Student Will describe the significance of Rutherford’s Gold Foil experiment on the discovery of the atomic nucleus. 2. TSW explain what determines the radii of the electron orbits in the Bohr model of the atom and its correlation to integral wavelengths in quantum mechanics. 3. TSW distinguis ...
... 1. The Student Will describe the significance of Rutherford’s Gold Foil experiment on the discovery of the atomic nucleus. 2. TSW explain what determines the radii of the electron orbits in the Bohr model of the atom and its correlation to integral wavelengths in quantum mechanics. 3. TSW distinguis ...
Atom Unit Review Questions File
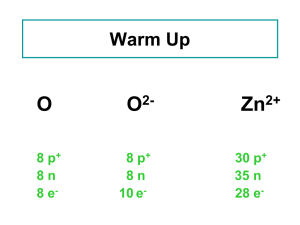
... 5. Isotopes are atoms of the same element that: a) have different numbers of electrons. b) have different numbers of protons. c) have different atomic numbers. d) have different numbers of neutrons. e) have different nuclear charges. ...
... 5. Isotopes are atoms of the same element that: a) have different numbers of electrons. b) have different numbers of protons. c) have different atomic numbers. d) have different numbers of neutrons. e) have different nuclear charges. ...
Rules for Naming Elements/Compounds
... – By definition, atoms have no overall electrical charge. That means that there must be a balance between the positively charged protons and the negatively charged electrons. Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it ...
... – By definition, atoms have no overall electrical charge. That means that there must be a balance between the positively charged protons and the negatively charged electrons. Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it ...
Chapter Review Answers
... Choose the answer that best completes each of the following sentences. Write the letter for that answer on the line to the left of each question. _______9. Each energy level of an atom has a maximum number of _____ it can hold. a. neutrons c. protons b. quarks d. electrons _______10. Dot diagrams ar ...
... Choose the answer that best completes each of the following sentences. Write the letter for that answer on the line to the left of each question. _______9. Each energy level of an atom has a maximum number of _____ it can hold. a. neutrons c. protons b. quarks d. electrons _______10. Dot diagrams ar ...
review-basics-atomic-structure-and-electron-configurations-v1
... Identify the person most associated with each statement below. Some names might be used more than once. a.) ____________ Gold Foil Experiment b.) ____________ Concept of electron shells c.) ____________ In noticing the ratios of elements are always constant for a given compound, concluded elements m ...
... Identify the person most associated with each statement below. Some names might be used more than once. a.) ____________ Gold Foil Experiment b.) ____________ Concept of electron shells c.) ____________ In noticing the ratios of elements are always constant for a given compound, concluded elements m ...
The Atom
... ** Also notice there is no change in mass. 5. Nuclide that decay by beta emission have too many neutrons in the nucleus for the number of protons present. ...
... ** Also notice there is no change in mass. 5. Nuclide that decay by beta emission have too many neutrons in the nucleus for the number of protons present. ...
Reading Comprehension - Easy Peasy All-in
... The list of elements is arranged on a scientific chart. The chart is called the periodic table. Each element is grouped with other similar elements. Elements can be metals, nonmetals, or semimetals. Semimetals have some of the characteristics of metals, and some of the characteristics of nonmetals. M ...
... The list of elements is arranged on a scientific chart. The chart is called the periodic table. Each element is grouped with other similar elements. Elements can be metals, nonmetals, or semimetals. Semimetals have some of the characteristics of metals, and some of the characteristics of nonmetals. M ...
Class 9 CBSE Test paper Solved Chapter 3: Structure of...
... and electronic configuration is 2,8,2. It can lose 2 electrons to get octet configuration thus its valency is 2. Oxygen has atomic number 8 and its electronic configuration is 2, 6. It can gain 2 electrons to get octet configuration thus its valency is 8-6=2 (iii) The atomic number is equal to numbe ...
... and electronic configuration is 2,8,2. It can lose 2 electrons to get octet configuration thus its valency is 2. Oxygen has atomic number 8 and its electronic configuration is 2, 6. It can gain 2 electrons to get octet configuration thus its valency is 8-6=2 (iii) The atomic number is equal to numbe ...
Atoms and their Structure
... century BC – world made up of empty space and tiny particles called atoms (atomos)‘indivisible’ – Hypothesized without using experiments ...
... century BC – world made up of empty space and tiny particles called atoms (atomos)‘indivisible’ – Hypothesized without using experiments ...
Isotopes-Chemistry
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
4-3-Isotopes
... Isotopes of Carbon Naturally occurring carbon consists of three isotopes, 12C, 13C, and 14C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12C ...
... Isotopes of Carbon Naturally occurring carbon consists of three isotopes, 12C, 13C, and 14C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12C ...
Name
... 11. Define the law of definite proportions. States that any sample of a compound always has the same composition. 12. Define the law of multiple proportions. States that elements always combine in simple whole number increments; ex) H2O & H2O2 13. Define the law of conservation of mass. States that ...
... 11. Define the law of definite proportions. States that any sample of a compound always has the same composition. 12. Define the law of multiple proportions. States that elements always combine in simple whole number increments; ex) H2O & H2O2 13. Define the law of conservation of mass. States that ...
1 Notes Ch. 4 and 25: Atomic Structure and Nuclear Chemistry
... II. Induced Transmutation • Before 1919, the only way to change the nucleus or cause transmutation was to wait for _______________________. • In 1919 Rutherford was the first to induce (_____________________) transmutation. • He proved that nuclear reactions can be produced _________________________ ...
... II. Induced Transmutation • Before 1919, the only way to change the nucleus or cause transmutation was to wait for _______________________. • In 1919 Rutherford was the first to induce (_____________________) transmutation. • He proved that nuclear reactions can be produced _________________________ ...
Review: theory vs law the atomic theory contributions of early scientists
... Neutron Heavy (similar to nucleus protons) Oct 711:36 AM ...
... Neutron Heavy (similar to nucleus protons) Oct 711:36 AM ...
Chapter 3 notes
... • Orbital- or energy shell/level is a region in an atom where there is a high probability of finding electrons. • Valence electron- an electron in the outermost energy level of an atom. So in ...
... • Orbital- or energy shell/level is a region in an atom where there is a high probability of finding electrons. • Valence electron- an electron in the outermost energy level of an atom. So in ...
atomic number - Net Start Class
... Synopsis of Structure • The atom is the smallest part of an element that retains its properties. • It is made of mostly empty space,with the majority of the mass concentrated in the middle (the nucleus). • The nucleus contains the positively charged protons and the chargeless neutrons. • The electr ...
... Synopsis of Structure • The atom is the smallest part of an element that retains its properties. • It is made of mostly empty space,with the majority of the mass concentrated in the middle (the nucleus). • The nucleus contains the positively charged protons and the chargeless neutrons. • The electr ...
Inside the Atom
... Some nuclei are unstable because they have too many or too few neutrons These unstable nuclei release particles to become more stable The release of nuclear particles and energy is called radioactive decay When particles like protons are emitted from nucleus, the Atomic # changes and a new a ...
... Some nuclei are unstable because they have too many or too few neutrons These unstable nuclei release particles to become more stable The release of nuclear particles and energy is called radioactive decay When particles like protons are emitted from nucleus, the Atomic # changes and a new a ...
Darlington High School EDI Lesson Plan Teacher: L. Grooms
... PS 2.5 Predict the charge that a representative element will acquire according to the arrangement of electrons in its outer energy level. Check for Understanding: ...
... PS 2.5 Predict the charge that a representative element will acquire according to the arrangement of electrons in its outer energy level. Check for Understanding: ...
Name - Aurora City Schools
... 46. – 47. There are a number of patterns in the periodic table. Name at least one as you go across the row and at least one as you go down a column. ...
... 46. – 47. There are a number of patterns in the periodic table. Name at least one as you go across the row and at least one as you go down a column. ...
Review Chemistry KEY - cms16-17
... The reactants are found before the arrow and the products are found after the arrow. 32. List each element in the following compounds and the number of atoms of each element present and the total number of atoms. a. C6H8O6 (Vitamin C): i. Elements: C, H, and O_____________________________________ ii ...
... The reactants are found before the arrow and the products are found after the arrow. 32. List each element in the following compounds and the number of atoms of each element present and the total number of atoms. a. C6H8O6 (Vitamin C): i. Elements: C, H, and O_____________________________________ ii ...
Name - Aurora City Schools
... 46. – 47. There are a number of patterns in the periodic table. Name at least one as you go across the row and at least one as you go down a column. ...
... 46. – 47. There are a number of patterns in the periodic table. Name at least one as you go across the row and at least one as you go down a column. ...
Neptunium
.png?width=300)
Neptunium is a chemical element with symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is radioactive, pyrophoric, and can accumulate in bones, which makes the handling of neptunium dangerous.Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then, most neptunium has been and still is produced by neutron irradiation of uranium in nuclear reactors. The vast majority is generated as a by-product in conventional nuclear power reactors. While neptunium itself has no commercial uses at present, it is widely used as a precursor for the formation of plutonium-238, used in radioisotope thermal generators. Neptunium has also been used in detectors of high-energy neutrons.The most stable isotope of neptunium, neptunium-237, is a by-product of nuclear reactors and plutonium production. It, and the isotope neptunium-239, are also found in trace amounts in uranium ores due to neutron capture reactions and beta decay.