Using your periodic table (9/30-10/6) File
... 1.Ions are a result of electrons being ________. 2. A positive charged element has ______ electrons & is called a ______. 3. A negative charged element has _____ electrons & is called a _____. 4. Draw the Bohr models of Na + Cl NaCl and show how electrons are transferred. Which part of the reactio ...
... 1.Ions are a result of electrons being ________. 2. A positive charged element has ______ electrons & is called a ______. 3. A negative charged element has _____ electrons & is called a _____. 4. Draw the Bohr models of Na + Cl NaCl and show how electrons are transferred. Which part of the reactio ...
Chocolate Challenge - Waterford Public Schools
... Protons determine element’s identity # of protons is unique for each element Electrons determine element’s chemical properties Neutrons act as a “glue” for the protons to minimize charge repulsions ...
... Protons determine element’s identity # of protons is unique for each element Electrons determine element’s chemical properties Neutrons act as a “glue” for the protons to minimize charge repulsions ...
Name Date: __ ______ Chemistry Semester I Final Exam Review
... The columns on the periodic table are called ________________. 36. Write the correct charge and name for the following ions. ...
... The columns on the periodic table are called ________________. 36. Write the correct charge and name for the following ions. ...
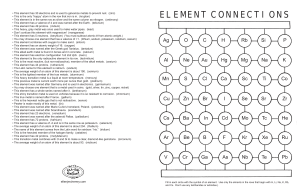
element connections
... • This element has a valence of 4 and was named after the Earth. (tellurium) • This element has 49 protons. (indium) • This heavy, gray metal was once used to make water pipes. (lead) • Don’t confuse this element with magnesium! (manganese) • This element has 5 neutrons. (beryllium) (You must subtra ...
... • This element has a valence of 4 and was named after the Earth. (tellurium) • This element has 49 protons. (indium) • This heavy, gray metal was once used to make water pipes. (lead) • Don’t confuse this element with magnesium! (manganese) • This element has 5 neutrons. (beryllium) (You must subtra ...
05: Atoms and Molecules
... neutrons. • amu: Atomic Mass Unit (1.66 × 10-27 kg) • Ion: Atom with a charge, resulting from the loss or gain of electrons. • Anion: Atom with a negative charge. • Cation: Atom with a positive charge. • Periodic Table: Organizes the elements. • Isotopes: Atoms of the same element with a different n ...
... neutrons. • amu: Atomic Mass Unit (1.66 × 10-27 kg) • Ion: Atom with a charge, resulting from the loss or gain of electrons. • Anion: Atom with a negative charge. • Cation: Atom with a positive charge. • Periodic Table: Organizes the elements. • Isotopes: Atoms of the same element with a different n ...
chpt 11 and 12 notes with answers
... ◦ Atom: Greek meaning “not able to be divide” Small hard particles that make up stuff (matter) Countered by Aristotle, stated never end up with something that cannot be divided ...
... ◦ Atom: Greek meaning “not able to be divide” Small hard particles that make up stuff (matter) Countered by Aristotle, stated never end up with something that cannot be divided ...
Chapter 4 Chemical Foundations: Elements, Atoms, and Ions
... Chapter 4 Chemical Foundations: Elements, Atoms, and Ions ...
... Chapter 4 Chemical Foundations: Elements, Atoms, and Ions ...
Atomic Structure
... Scientist use units known as Atomic mass units (amu) A proton or a neutron has a mass equal to about 1/1000th Atomic Mass is equal to the number of protons and neutrons in an atom. ...
... Scientist use units known as Atomic mass units (amu) A proton or a neutron has a mass equal to about 1/1000th Atomic Mass is equal to the number of protons and neutrons in an atom. ...
2.2 Periodic Trends
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
Activity 2 - SSS Chemistry
... ________________________________________________________________________ ________________________________________________________________________ ...
... ________________________________________________________________________ ________________________________________________________________________ ...
atoms and elements
... Atoms and Elements An atom is the smallest particle into which an element can be divided and still maintain the properties of that element. All elements are made of atoms. So what’s an element? What makes one element different from another? Let’s find out! Vocabulary: First things first, let’s look ...
... Atoms and Elements An atom is the smallest particle into which an element can be divided and still maintain the properties of that element. All elements are made of atoms. So what’s an element? What makes one element different from another? Let’s find out! Vocabulary: First things first, let’s look ...
Atomic Theory and Structure Notes
... something you want to explain (so a model that represents the structure of an atom is called an atomic model). ...
... something you want to explain (so a model that represents the structure of an atom is called an atomic model). ...
Review Questions 1. How many protons does potassium have? 2
... b. Nuclear forces equalize the charges c. The number of protons and electrons is equal d. The number of protons and neutrons is equal 31. The most common form of hydrogen has a. No neutrons b. 1 neutron c. 2 neutrons d. 3 neutrons 32. The name of the scientist who showed the existence of the nucleus ...
... b. Nuclear forces equalize the charges c. The number of protons and electrons is equal d. The number of protons and neutrons is equal 31. The most common form of hydrogen has a. No neutrons b. 1 neutron c. 2 neutrons d. 3 neutrons 32. The name of the scientist who showed the existence of the nucleus ...
Atomic Structure Subatomic Particles Atoms are made up of even
... Atomic Number (Z) and Mass Number (M) The atomic number is what determines the atom’s identity. Atomic number = number of protons in an atom For atoms that are electrically neutral, the number of protons = the number of electrons For ions, the number of protons ≠ the number of electrons Cations have ...
... Atomic Number (Z) and Mass Number (M) The atomic number is what determines the atom’s identity. Atomic number = number of protons in an atom For atoms that are electrically neutral, the number of protons = the number of electrons For ions, the number of protons ≠ the number of electrons Cations have ...
Chapter 2: Atoms, Molecules, and Ions
... Exam Problem. A sample of ascorbic acid (vitamin C) is synthesized in the laboratory. It contains 1.50 g of carbon and 2.00 g of oxygen. Another sample of ascorbic acid isolated from citrus fruits contains 9.22 g of carbon. How many grams of oxygen does it contain? ...
... Exam Problem. A sample of ascorbic acid (vitamin C) is synthesized in the laboratory. It contains 1.50 g of carbon and 2.00 g of oxygen. Another sample of ascorbic acid isolated from citrus fruits contains 9.22 g of carbon. How many grams of oxygen does it contain? ...
Atomic Structure - Mr. Cervantes Science Classes
... reasonable to think that the mass of an atom should be expressed as a whole number B. The atomic mass of an element is the weighted average of the masses of all the isotopes of that element 1. When calculating the average atomic mass you must take into account the relative abundance of each isotope ...
... reasonable to think that the mass of an atom should be expressed as a whole number B. The atomic mass of an element is the weighted average of the masses of all the isotopes of that element 1. When calculating the average atomic mass you must take into account the relative abundance of each isotope ...
Protons, electrons and neutrons worksheet
... Atomic symbol is the symbol you find for each element shown in the periodic table. Magnesium symbol is Mg Gold symbol is Au Potassium symbol is K Phosphorous symbol is P Note: First letter of the element is not always the symbol. Atomic number is the number on the top left of atomic symbol in period ...
... Atomic symbol is the symbol you find for each element shown in the periodic table. Magnesium symbol is Mg Gold symbol is Au Potassium symbol is K Phosphorous symbol is P Note: First letter of the element is not always the symbol. Atomic number is the number on the top left of atomic symbol in period ...
Name___________________________________ Physical
... B) Protons are positively charged and the lightest subatomic particle. C) The mass of a neutron nearly equals the mass of a proton. D) Electrons are negatively charged and are the heaviest subatomic particle. E) Neutrons have no charge and are the lightest subatomic particle. ...
... B) Protons are positively charged and the lightest subatomic particle. C) The mass of a neutron nearly equals the mass of a proton. D) Electrons are negatively charged and are the heaviest subatomic particle. E) Neutrons have no charge and are the lightest subatomic particle. ...
Neptunium
.png?width=300)
Neptunium is a chemical element with symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is radioactive, pyrophoric, and can accumulate in bones, which makes the handling of neptunium dangerous.Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then, most neptunium has been and still is produced by neutron irradiation of uranium in nuclear reactors. The vast majority is generated as a by-product in conventional nuclear power reactors. While neptunium itself has no commercial uses at present, it is widely used as a precursor for the formation of plutonium-238, used in radioisotope thermal generators. Neptunium has also been used in detectors of high-energy neutrons.The most stable isotope of neptunium, neptunium-237, is a by-product of nuclear reactors and plutonium production. It, and the isotope neptunium-239, are also found in trace amounts in uranium ores due to neutron capture reactions and beta decay.