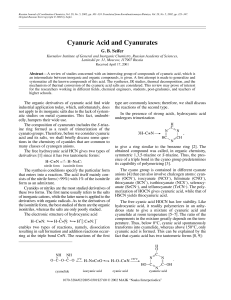
Cyanuric Acid and Cyanurates
... The entropy changes and the heat effect of the HCN polymerization are calculated in [22], while the magnetic anisotropy and the charge delocalization in S-triazine are considered in [23]. The IR spectrum of the polymerized HCN is given in [3] (ν, cm–1): 3450, 3370, 3314, 3260, 3219, 3184 ν(NH2); 222 ...
... The entropy changes and the heat effect of the HCN polymerization are calculated in [22], while the magnetic anisotropy and the charge delocalization in S-triazine are considered in [23]. The IR spectrum of the polymerized HCN is given in [3] (ν, cm–1): 3450, 3370, 3314, 3260, 3219, 3184 ν(NH2); 222 ...
Cyanide Destruction with Chlorine Dioxide
... pH to avoid cyanogen chloride formation. The presence of ammonia will also create a high demand for chlorine. Hydrogen peroxide is the most common oxidant, but requires high pH (through caustic addition) and is much more costly than alkaline chlorination. ...
... pH to avoid cyanogen chloride formation. The presence of ammonia will also create a high demand for chlorine. Hydrogen peroxide is the most common oxidant, but requires high pH (through caustic addition) and is much more costly than alkaline chlorination. ...
File
... Page 3 of 114 sig figs and always include proper units. Underline, use capital letters or use any device you choose to help organize this section well. Space things out – don’t try to cram everything on one page. A data table must have a label and a title. e.g. – Table 1: Density Values for Sugar So ...
... Page 3 of 114 sig figs and always include proper units. Underline, use capital letters or use any device you choose to help organize this section well. Space things out – don’t try to cram everything on one page. A data table must have a label and a title. e.g. – Table 1: Density Values for Sugar So ...
Multiple-choice questions : 1. Which of the following solutions
... Each question below consists of two separate statements. Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the following table ...
... Each question below consists of two separate statements. Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the following table ...
Chemistry 134 Problem Set Introduction
... 14.38 (a) What is the difference between a sapphire and a ruby? (b) Why might aluminum be present with silicon in many minerals? 14.39 (a) List the stable oxidation states for each member of the boron family. (b) For any element that may have more than one stable oxidation state, identify the more s ...
... 14.38 (a) What is the difference between a sapphire and a ruby? (b) Why might aluminum be present with silicon in many minerals? 14.39 (a) List the stable oxidation states for each member of the boron family. (b) For any element that may have more than one stable oxidation state, identify the more s ...
Chapter 4 Chemical Reactions and Solution Stoichiometry 4.1
... When mixed, a double displacement reaction takes place, forming the soluble compound NaNO3 and the insoluble compound AgCl. In the reaction vessel the Ag+ and Cl ions combine, and a white solid precipitates from the solution. As the solid precipitates, the Na+ and NO3 ions remain in solution. The ...
... When mixed, a double displacement reaction takes place, forming the soluble compound NaNO3 and the insoluble compound AgCl. In the reaction vessel the Ag+ and Cl ions combine, and a white solid precipitates from the solution. As the solid precipitates, the Na+ and NO3 ions remain in solution. The ...
SYLLABUS 5070 Cambridge O Level Chemistry
... (a) name appropriate apparatus for the measurement of time, temperature, mass and volume, including burettes, pipettes, measuring cylinders and gas syringes (b) suggest suitable apparatus, given relevant information, for a variety of simple experiments, including collection of gases and measurement ...
... (a) name appropriate apparatus for the measurement of time, temperature, mass and volume, including burettes, pipettes, measuring cylinders and gas syringes (b) suggest suitable apparatus, given relevant information, for a variety of simple experiments, including collection of gases and measurement ...
Acids ,Bases and Salts
... This dissociation/ionization makes aqueous ammonia to: (i)turn litmus paper/solution blue. (ii)have pH 8/9/10/11 (iii)be a good electrical conductor (iv)react with acids to form ammonium salt and water only. NH4OH(aq) + HCl(aq) -> NH4Cl(aq) + H2O(l) (d)Ammonia gas dissolves in methylbenzene/benzene ...
... This dissociation/ionization makes aqueous ammonia to: (i)turn litmus paper/solution blue. (ii)have pH 8/9/10/11 (iii)be a good electrical conductor (iv)react with acids to form ammonium salt and water only. NH4OH(aq) + HCl(aq) -> NH4Cl(aq) + H2O(l) (d)Ammonia gas dissolves in methylbenzene/benzene ...
1.24 calculations and chemical reactions
... 5.1) A 2.23g sample of magnesium nitrate was fully decomposed. 2 Mg(NO3)2 2MgO + 4NO2 + O2 The magnesium oxide, produced was reacted with hydrochloric acid. MgO + 2HCl MgCl2 + H2O This sample of magnesium oxide required 33.2cm3 of hydrochloric acid for complete reaction. Calculate the concentration, ...
... 5.1) A 2.23g sample of magnesium nitrate was fully decomposed. 2 Mg(NO3)2 2MgO + 4NO2 + O2 The magnesium oxide, produced was reacted with hydrochloric acid. MgO + 2HCl MgCl2 + H2O This sample of magnesium oxide required 33.2cm3 of hydrochloric acid for complete reaction. Calculate the concentration, ...
Word Pro
... This is the contents of a Quiz 1 from a few years ago in the days of Chemistry 1000. (It has been reformatted to save paper) Answer ALL of the questions in the spaces provided. The mark that you obtain for this test will be used in calculating your final grade for the course. 1. Name the following c ...
... This is the contents of a Quiz 1 from a few years ago in the days of Chemistry 1000. (It has been reformatted to save paper) Answer ALL of the questions in the spaces provided. The mark that you obtain for this test will be used in calculating your final grade for the course. 1. Name the following c ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.