text
... chemist who is studying the effect of pollution on spruce trees needs to know, or know where to find, the chemical differences between p‑hydroxybenzoic acid and p‑hydroxyacetophenone, two common phenols found in the needles of spruce trees. The ability to “think as a chemist” is a product of your ex ...
... chemist who is studying the effect of pollution on spruce trees needs to know, or know where to find, the chemical differences between p‑hydroxybenzoic acid and p‑hydroxyacetophenone, two common phenols found in the needles of spruce trees. The ability to “think as a chemist” is a product of your ex ...
analytical chemistry - Львівський національний медичний
... selection of reaction conditions. By changing these conditions, we can increase or decrease the yield of product. We might change the yield by: 1. Changing concentrations by removing products or adding reactants to the reaction vessel. 2. Changing the partial pressure of gaseous reactants and produc ...
... selection of reaction conditions. By changing these conditions, we can increase or decrease the yield of product. We might change the yield by: 1. Changing concentrations by removing products or adding reactants to the reaction vessel. 2. Changing the partial pressure of gaseous reactants and produc ...
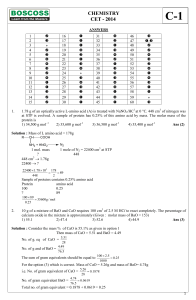
CHEMISTRY CET
... An incorrect statement with respect to SN1 and SN2 mechanisms for alkyl halide is (1) Competing reaction for an SN2 reaction is rearrangement. (2) A weak nucleophile and a protic solvent increases the rate or favours SN1 reaction. (3) A strong nucleophile in an aprotic solvent increases the rate or ...
... An incorrect statement with respect to SN1 and SN2 mechanisms for alkyl halide is (1) Competing reaction for an SN2 reaction is rearrangement. (2) A weak nucleophile and a protic solvent increases the rate or favours SN1 reaction. (3) A strong nucleophile in an aprotic solvent increases the rate or ...
5. Coenzyme HAD+ is derived
... 3 Independent work of students under the guidance of a teacher: Working in small groups, the solution situational tasks, consultations, discussions, presentations, essays, performance tests, discuss the results of individual and group assignments. 4 Self-study students work with literature, electron ...
... 3 Independent work of students under the guidance of a teacher: Working in small groups, the solution situational tasks, consultations, discussions, presentations, essays, performance tests, discuss the results of individual and group assignments. 4 Self-study students work with literature, electron ...
BARIUM NITRATE
... Precipitated BaSO4 is often used in many industrial applications. Blanc fixe and Lithopone are made by the reactions of barium sulfide with sodium sulfate and zinc sulfate, respectively. Reactions Barium sulfate is one of the most insoluble salts of barium. It does not undergo double decomposition r ...
... Precipitated BaSO4 is often used in many industrial applications. Blanc fixe and Lithopone are made by the reactions of barium sulfide with sodium sulfate and zinc sulfate, respectively. Reactions Barium sulfate is one of the most insoluble salts of barium. It does not undergo double decomposition r ...
Chemistry of CHLORINE
... Iron glows red hot Brown crystals are formed Explanation Iron reacts with chlorine to form dark brown crystals of iron (III) Chloride. This reaction is exothermic and requires no farther heating once started. Iron (III) Chloride sublimes away ensuring the unreacted Iron completely reacts with chlori ...
... Iron glows red hot Brown crystals are formed Explanation Iron reacts with chlorine to form dark brown crystals of iron (III) Chloride. This reaction is exothermic and requires no farther heating once started. Iron (III) Chloride sublimes away ensuring the unreacted Iron completely reacts with chlori ...
Boronic acids facilitate rapid oxime condensations at neutral pH
... condensation is the size of required 2-FBPA motif. Although for most applications this should present no difficulties, examples where the compactness of the oxime is critical (such as, for example, as a functional isostere of peptide bonds)37 would not be possible. The ability to run conjugations at 1 ...
... condensation is the size of required 2-FBPA motif. Although for most applications this should present no difficulties, examples where the compactness of the oxime is critical (such as, for example, as a functional isostere of peptide bonds)37 would not be possible. The ability to run conjugations at 1 ...
File - UTeach Dallas Project
... emphasis on factual material and greater emphasis on understanding and application of scientific concepts and principles. This has been done so that learners develop skills that will be of the value for a long time in an increasingly world and it is expected that these will be of relevance for a ver ...
... emphasis on factual material and greater emphasis on understanding and application of scientific concepts and principles. This has been done so that learners develop skills that will be of the value for a long time in an increasingly world and it is expected that these will be of relevance for a ver ...
Exam 1
... As the mixture was heated the temperature shown by the thermometer initially rose but then remained constant at 78°C for some time. Which one of the following statements about percentage of ethanol in the vapours shown at points X, Y and Z, when the temperature is at a constant 78°C, is true? A. The ...
... As the mixture was heated the temperature shown by the thermometer initially rose but then remained constant at 78°C for some time. Which one of the following statements about percentage of ethanol in the vapours shown at points X, Y and Z, when the temperature is at a constant 78°C, is true? A. The ...
Dr. Spencer`s PPT
... Nonelectrolytes are not dissociated into ions in solution Extent of dissolution does not dictate strong or weak electrolyte solution (i.e., HC2H3O2 is very soluble but is a weak electrolyte while Ba(OH)2 is only slightly soluble is a strong electrolyte) ...
... Nonelectrolytes are not dissociated into ions in solution Extent of dissolution does not dictate strong or weak electrolyte solution (i.e., HC2H3O2 is very soluble but is a weak electrolyte while Ba(OH)2 is only slightly soluble is a strong electrolyte) ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.